Improved Physicochemical Properties of Yogurt Fortified with Fish Oil/γ-Oryzanol by Nanoemulsion Technology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production of Fish Oil/γ-Oryzanol Nanoemulsion
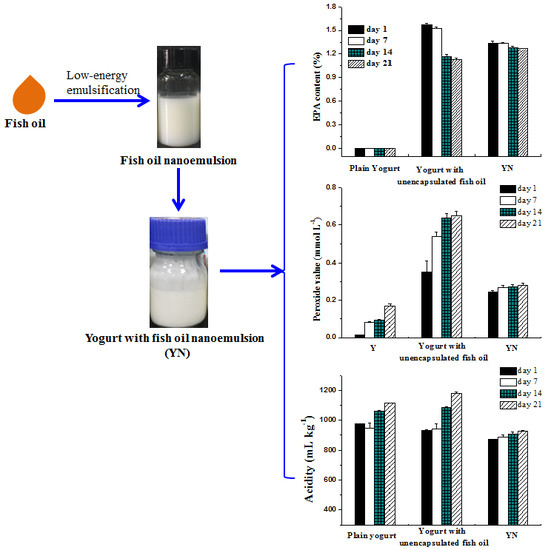
2.2. Effect of Adding Fish Oil/γ-Oryzanol Nanoemulsion on Physicochemical Properties of Yogurt
2.2.1. pH and Acidity
2.2.2. Viscosity of Yogurts
2.2.3. Syneresis of Yogurt Samples
2.2.4. Fatty Acid Composition of Yogurts
2.2.5. Peroxide Value of Yogurts
2.2.6. Melting and Crystallization Behavior of Yogurts
2.3. Sensory Evaluation of Yogurts
3. Materials and Methods
3.1. Materials
3.2. Production of Fish Oil/γ-Oryzanol Nanoemulsion and Its Particle Size Measurement
3.3. Preparation of Yogurt Fortified with Fish Oil/γ-Oryzanol Nanoemulsion
3.4. Physicochemical Properties of Yogurt Samples
3.4.1. Acidity and pH
3.4.2. Syneresis
3.4.3. Viscosity Property
3.4.4. Chemical Stability Testing
3.4.5. Freezing and Melting Behavior of Yogurts
3.5. Sensory Evaluation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kromhout, D.; de Goede, J. Update on cardiometabolic health effects of ω-3 fatty acids. Curr. Opin. Lipidol. 2014, 25, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Nik, A.M.; Corredig, M.; Wright, A.J. Release of lipophilic molecules during in vitro digestion of soy protein-stabilized emulsions. Mol. Nutr. Food Res. 2011, 55, S278–S289. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, W.; Swiderski, F.; Lis, E.; Berger, S. Enrichment of spreadable fats with polyunsaturated fatty acids omega-3 using fish oil. Int. J. Food Sci. Nutr. 2001, 52, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Lyu, Y.; Narsimhan, G. Characterization of fish oil in water emulsion produced by layer by layer deposition of soy β-conglycinin and high methoxyl pectin. Food Hydrocoll. 2016, 52, 678–689. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Lin, L. Characterization, stability, and in vitro release evaluation of carboxymethyl chitosan coated liposomes containing fish oil. J. Food Sci. 2015, 80, C1460–C1467. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.C.; Rojas-Graü, M.A.; Martín-Belloso, O.; McClements, D.J. Edible nanoemulsions as carriers of active ingredients: A review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef] [PubMed]
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical properties and storage stability of ultrasound-mediated WPI-stabilized fish oil nanoemulsions. Food Hydrocoll. 2016, 61, 801–811. [Google Scholar] [CrossRef]
- Neves, M.A.; Wang, Z.; Kobayashi, I.; Nakajima, M. Assessment of oxidative stability in fish oil-in-water emulsions: Effect of emulsification process, droplet size and storage temperature. J. Food Process Eng. 2015. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schwarz, K. Chemical stabilisation of oils rich in long-chain polyunsaturated fatty acids during homogenisation, microencapsulation and storage. Food Chem. 2009, 113, 1106–1112. [Google Scholar] [CrossRef]
- Komaiko, J.S.; McClements, D.J. Formation of food-grade nanoemulsions using low-energy preparation methods: A review of available methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Walker, R.M.; Decker, E.A.; McClements, D.J. Physical and oxidative stability of fish oil nanoemulsions produced by spontaneous emulsification: Effect of surfactant concentration and particle size. J. Food Eng. 2015, 164, 10–20. [Google Scholar] [CrossRef]
- Uluata, S.; McClements, D.J.; Decker, E.A. Physical stability, autoxidation, and photosensitized oxidation of ω-3 oils in nanoemulsions prepared with natural and synthetic surfactants. J. Agric. Food Chem. 2015, 63, 9333–9340. [Google Scholar] [CrossRef] [PubMed]
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical properties and oxidative stability of fish oil nanoemulsions as affected by hydrophilic lipophilic balance, surfactant to oil ratio and storage temperature. Colloids Surf. A 2016, 506, 821–832. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Lerma-Garcia, M.; Herrero-Martinez, J.; Simó-Alfonso, E.; Mendonça, C.R.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, X.; Wang, Y.; Qin, X.; Li, Z. γ-Oryzanol nanoemulsions produced by low-energy emulsification method: Evaluation of process parameters and physicochemical stability. Food Funct. 2017, 8, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Chanamai, R.; McClements, D.J. Comparison of gum arabic, modified starch, and whey protein isolate as emulsifiers: Influence of pH, CaCl2 and temperature. J. Food Sci. 2002, 67, 120–125. [Google Scholar] [CrossRef]
- Cai, C.; Pan, S.Y. Prediction Model of Shelf Life of Yogurt to Various Parameters during Storage. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2012. [Google Scholar]
- Cheng, J.; Xie, S.; Yin, Y.; Feng, X.; Wang, S.; Guo, M.; Ni, C. Physiochemical, texture properties, and the microstructure of set yogurt using whey protein-sodium tripolyphosphate aggregates as thickening agents. J. Sci. Food Agric. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bakirci, S.; Dagdemir, E.; Boran, O.S.; Hayaloglu, A.A. The effect of pumpkin fibre on quality and storage stability of reduced-fat set-type yogurt. Int. J. Food. Sci. Technol. 2017, 52, 180–187. [Google Scholar] [CrossRef]
- Delikanli, B.; Ozcan, T. Improving the textural properties of yogurt fortified with milk proteins. J. Food Process Preserv. 2017, 41, e13101. [Google Scholar] [CrossRef]
- Costa, M.P.; Frasao, B.S.; Rodrigues, B.L.; Silva, A.C.; Conte-Junior, C.A. Effect of different fat replacers on the physicochemical and instrumental analysis of low-fat cupuassu goat milk yogurts. J. Dairy Res. 2016, 83, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Cui, J.; Taneja, A.; Zhu, X.; Singh, H. Evaluation of processed cheese fortified with fish oil emulsion. Food Res. Int. 2009, 42, 1093–1098. [Google Scholar] [CrossRef]
- The Ministry of Health of the People’s Republic of China. GB 5413.34-2010. Determination of Acidity in Milk and Milk Products; The Ministry of Health of the People’s Republic of China: Beijing, China, 2010.
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [PubMed]
- Alfaro, L.; Hayes, D.; Boeneke, C.; Xu, Z.; Bankston, D.; Bechtel, P.J.; Sathivel, S. Physical properties of a frozen yogurt fortified with a nano-emulsion containing purple rice bran oil. LWT-Food Sci. Technol. 2015, 62, 1184–1191. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not are available from the authors. |




| Sample † | Overall Enthalpy (J g−1) | Extrapolated Onset Temperature (°C) | Endothermic Peak Temperature (°C) |
|---|---|---|---|
| Y-day 1 | 257.69 ± 28.55 a | −2.72 ± 0.08 a | 3.58 ± 0.01 a |
| YF-day 1 | 349.55 ± 29.47 a | −2.73 ± 0.24 a | 3.62 ± 0.45 a |
| YN-day 1 | 245.42 ± 17.35 a | −2.35 ± 0.14 a | 3.31 ± 0.23 a |
| Yogurt Samples † | Sensory Quality Score | |||
|---|---|---|---|---|
| Color | Taste and Aroma | Texture | Overall Acceptance | |
| Y-day 1 | 4.7 ± 0.5 a | 4.5 ± 0.5 a | 4.5 ± 0.5 a | 4.6 ± 0.5 a |
| YF-day 1 | 4.1 ± 0.3 b | 1.3 ± 0.5 c | 3.7 ± 0.5 c | 2.2 ± 0.5 c |
| YN-day 1 | 4.3 ± 0.4 b | 2.7 ± 0.5 b | 4.1 ± 0.4 b | 3.5 ± 0.6 b |
| Y-day 15 | 4.9 ± 0.3 a | 4.8 ± 0.4 a | 3.7 ± 0.7 a | 4.4 ± 0.5 a |
| YF-day 15 | 4.1 ± 0.2 b | 1.4 ± 0.6 c | 3.6 ± 0.6 a | 2.1 ± 0.6 b |
| YN-day 15 | 4.2 ± 0.4 b | 2.1 ± 0.6 b | 3.8 ± 0.4 a | 3.2 ± 0.5 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Yang, R.; Cao, X.; Liu, X.; Qin, X. Improved Physicochemical Properties of Yogurt Fortified with Fish Oil/γ-Oryzanol by Nanoemulsion Technology. Molecules 2018, 23, 56. https://doi.org/10.3390/molecules23010056
Zhong J, Yang R, Cao X, Liu X, Qin X. Improved Physicochemical Properties of Yogurt Fortified with Fish Oil/γ-Oryzanol by Nanoemulsion Technology. Molecules. 2018; 23(1):56. https://doi.org/10.3390/molecules23010056
Chicago/Turabian StyleZhong, Jinfeng, Rong Yang, Xiaoyi Cao, Xiong Liu, and Xiaoli Qin. 2018. "Improved Physicochemical Properties of Yogurt Fortified with Fish Oil/γ-Oryzanol by Nanoemulsion Technology" Molecules 23, no. 1: 56. https://doi.org/10.3390/molecules23010056
APA StyleZhong, J., Yang, R., Cao, X., Liu, X., & Qin, X. (2018). Improved Physicochemical Properties of Yogurt Fortified with Fish Oil/γ-Oryzanol by Nanoemulsion Technology. Molecules, 23(1), 56. https://doi.org/10.3390/molecules23010056