Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines
Abstract
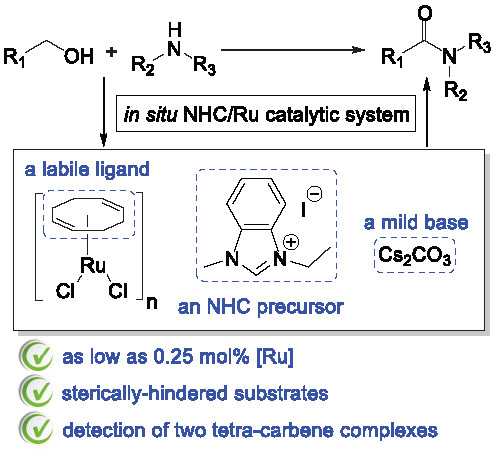
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General Considerations
3.2. General Procedure for the Amide Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Humphrey, J.M.; Chamberlin, A.R. Chemical synthesis of natural product peptides: Coupling methods for the incorporation of noncoded amino acids into peptides. Chem. Rev. 1997, 97, 2243–2266. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.W. Emerging methods in amide- and peptide-bond formation. Curr. Opin. Drug Discov. Dev. 2006, 9, 765–775. [Google Scholar] [CrossRef]
- Cupido, T.; Tulla-Puche, J.; Spengler, J.; Albericio, F. The synthesis of naturally occurring peptides and their analogs. Curr. Opin. Drug Discov. Dev. 2007, 10, 768–783. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Punniyamurthy, T. Palladium-catalyzed one-pot conversion of aldehydes to amides. Adv. Synth. Catal. 2010, 352, 288–292. [Google Scholar] [CrossRef]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, Y.A. Recent development of peptide coupling reagents in organic synthesis. Tetrahedron 2004, 60, 2447–2467. [Google Scholar] [CrossRef]
- Kohn, M.; Breinbauer, R. The Staudinger ligation-A gift to chemical biology. Angew. Chem. Int. Ed. 2004, 43, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Kolakowski, R.V.; Shangguan, N.; Sauers, R.R.; Williams, L.J. Mechanism of thio acid/azide amidation. J. Am. Chem. Soc. 2006, 128, 5695–5702. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Murphy, J.A. Azide rearrangements in electron-deficient systems. Chem. Soc. Rev. 2006, 35, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, J.R.; Clark, T.P.; Watson, D.A.; Munday, R.H.; Buchwald, S.L. Palladium-catalyzed aminocarbonylation of aryl chlorides at atmospheric pressure: The dual role of sodium phenoxide. Angew. Chem. Int. Ed. 2007, 46, 8460–8463. [Google Scholar] [CrossRef] [PubMed]
- Owston, N.A.; Parker, A.J.; Williams, J.M.J. Iridium-catalyzed conversion of alcohols into amides via oximes. Org. Lett. 2007, 9, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.W.; Chan, P.W.H. Highly efficient ruthenium (II) porphyrin catalyzed amidation of aldehydes. Angew. Chem. Int. Ed. 2008, 47, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Constable, D.J.C.; Dunn, P.J.; Hayler, J.D.; Humphrey, G.R.; Leazer, J.L., Jr.; Linderman, R.J.; Lorenz, K.; Manley, J.; Pearlman, B.A.; Wells, A.; et al. Key green chemistry research areas-a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. [Google Scholar] [CrossRef]
- Allen, C.L.; Williams, J.M.J. Metal-catalysed approaches to amide bond formation. Chem. Soc. Rev. 2011, 40, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hong, S.H. Oxidative amide synthesis directly from alcohols with amines. Org. Biomol. Chem. 2011, 9, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Applications of acceptorless dehydrogenation and related transformations in chemical synthesis. Science 2013. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Bond activation and catalysis by ruthenium pincer complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, R.M.; Suppo, J.S.; Campagne, J.M. Nonclassical routes for amide bond formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Q.; Fan, G.M.; Zhu, R.J.; Shi, L.; Xiao, S.Y.; Bi, C. Highly efficient synthesis of amides. Prog. Chem. 2016, 28, 497–506. [Google Scholar]
- Gusey, D.G. Rethinking the dehydrogenative amide synthesis. ACS Catal. 2017, 7, 6656–6662. [Google Scholar]
- Chen, C.; Verpoort, F.; Wu, Q.Y. Atom-economic dehydrogenative amide synthesis via ruthenium catalysis. RSC Adv. 2016, 6, 55599–55607. [Google Scholar] [CrossRef]
- Naota, T.; Murahashi, S.I. Ruthenium-catalyzed transformations of amino-alcohols to lactams. Synlett 1991, 10, 693–694. [Google Scholar] [CrossRef]
- Gunanathan, C.; Ben-David, Y.; Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H-2. Science 2007, 317, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, B.; Balaraman, E.; Ben-David, Y.; Milstein, D. Synthesis of peptides and pyrazines from β-Amino alcohols through extrusion of H2 catalyzed by ruthenium pincer complexes: Ligand-controlled selectivity. Angew. Chem. Int. Ed. 2011, 50, 12240–12244. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, B.; Balaraman, E.; Gunanathan, C.; Milstein, D. Synthesis of polyamides from diols and diamines with liberation of H2. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1755–1765. [Google Scholar] [CrossRef]
- Srimani, D.; Balaraman, E.; Hu, P.; Ben-David, Y.; Milstein, D. Formation of tertiary amides and dihydrogen by dehydrogenative coupling of primary alcohols with secondary amines catalyzed by ruthenium bipyridine-based pincer complexes. Adv. Synth. Catal. 2013, 355, 2525–2530. [Google Scholar] [CrossRef]
- Nordstrøm, L.U.; Vogt, H.; Madsen, R. Amide synthesis from alcohols and amines by the extrusion of dihydrogen. J. Am. Chem. Soc. 2008, 130, 17672–17673. [Google Scholar] [CrossRef] [PubMed]
- Dam, J.H.; Osztrovszky, G.; Nordstrøm, L.U.; Madsen, R. Amide synthesis from alcohols and amines catalyzed by ruthenium N-heterocyclic carbene complexes. Chem. Eur. J. 2010, 16, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Makarov, I.S.; Fristrup, P.; Madsen, R. Mechanistic investigation of the ruthenium-N-heterocyclic-carbene-catalyzed amidation of amines with alcohols. Chem. Eur. J. 2012, 18, 15683–15692. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Maxwell, A.C.; Williams, J.M.J. Ruthenium-catalyzed oxidation of alcohols into amides. Org. Lett. 2009, 11, 2667–2670. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Wakeham, R.J.; Maxwell, A.C.; Williams, J.M.J. Ruthenium-catalysed oxidation of alcohols to amides using a hydrogen acceptor. Tetrahedron 2014, 70, 3683–3690. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Muthaiah, S.; Zhang, Y.; Xu, X.Y.; Hong, S.H. Direct amide synthesis from alcohols and amines by phosphine-free ruthenium catalyst systems. Adv. Synth. Catal. 2009, 351, 2643–2649. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Ghosh, S.C.; Li, Y.X.; Hong, S.H. Well-defined N-heterocyclic carbene based ruthenium catalysts for direct amide synthesis from alcohols and amines. Organometallics 2010, 29, 1374–1378. [Google Scholar] [CrossRef]
- Muthaiah, S.; Ghosh, S.C.; Jee, J.E.; Chen, C.; Zhang, J.; Hong, S.H. Direct amide synthesis from either alcohols or aldehydes with amines: Activity of Ru (II) hydride and Ru (0) complexes. J. Org. Chem. 2010, 75, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.C.; Hong, S.H. Simple RuCl3-catalyzed amide synthesis from alcohols and amines. Eur. J. Org. Chem. 2010, 4266–4270. [Google Scholar] [CrossRef]
- Zhang, J.; Senthilkumar, M.; Ghosh, S.C.; Hong, S.H. Synthesis of cyclic imides from simple diols. Angew. Chem. Int. Ed. 2010, 49, 6391–6395. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Hong, S.H. N-heterocyclic carbene based ruthenium-catalyzed direct amide synthesis from alcohols and secondary amines: Involvement of esters. J. Org. Chem. 2011, 76, 10005–10010. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hong, S.H. Selective catalytic sp3 C-O bond cleavage with C-N bond formation in 3-alkoxy-1-propanols. Org. Lett. 2012, 14, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kang, B.; Hong, S.H. N-Heterocyclic carbene-based well-defined ruthenium hydride complexes for direct amide synthesis from alcohols and amines under base-free conditions. Tetrahedron 2015, 71, 4565–4569. [Google Scholar] [CrossRef]
- Kang, B.; Hong, S.H. Hydrogen acceptor- and base-free N-formylation of nitriles and amines using methanol as C-1 Source. Adv. Synth. Catal. 2015, 357, 834–840. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.H. Ruthenium-catalyzed urea synthesis using methanol as the C1 source. Org. Lett. 2016, 18, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Nova, A.; Balcells, D.; Schley, N.D.; Dobereiner, G.E.; Crabtree, R.H.; Eisenstein, O. An experimental-theoretical study of the factors that affect the switch between ruthenium-catalyzed dehydrogenative amide formation versus amine alkylation. Organometallics 2010, 29, 6548–6558. [Google Scholar] [CrossRef]
- Schley, N.D.; Dobereiner, G.E.; Crabtree, R.H. Oxidative synthesis of amides and pyrroles via dehydrogenative alcohol oxidation by ruthenium diphosphine diamine complexes. Organometallics 2011, 30, 4174–4179. [Google Scholar] [CrossRef]
- Prades, A.; Peris, E.; Albrecht, M. Oxidations and oxidative couplings catalyzed by triazolylidene ruthenium complexes. Organometallics 2011, 30, 1162–1167. [Google Scholar] [CrossRef]
- Zeng, H.; Guan, Z. Direct synthesis of polyamides via catalytic dehydrogenation of diols and diamines. J. Am. Chem. Soc. 2011, 133, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Oldenhuis, N.J.; Dong, V.M.; Guan, Z. Catalytic acceptorless dehydrogenations: Ru-Macho catalyzed construction of amides and imines. Tetrahedron 2014, 70, 4213–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, N.; Richter, C.; Glorius, F. N-formylation of amines by methanol activation. Org. Lett. 2013, 15, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Malineni, J.; Merkens, C.; Keul, H.; Möller, M. An efficient N-heterocyclic carbene based ruthenium-catalyst: Application towards the synthesis of esters and amides. Catal. Commun. 2013, 40, 80–83. [Google Scholar] [CrossRef]
- Malineni, J.; Keul, H.; Möller, M. An efficient N-heterocyclic carbene-ruthenium complex: Application towards the synthesis of polyesters and polyamides. Macromol. Rapid Commun. 2015, 36, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Sengupta, G.; Sarbajna, A.; Dutta, I.; Bera, J.K. Amide synthesis from alcohols and amines catalyzed by a Ru-II-N-heterocyclic carbene (NHC)-carbonyl complex. J. Organomet. Chem. 2014, 771, 124–130. [Google Scholar] [CrossRef]
- Xie, X.K.; Huynh, H.V. Tunable dehydrogenative amidation versus amination using a single ruthenium-NHC catalyst. ACS Catal. 2015, 5, 4143–4151. [Google Scholar] [CrossRef]
- Nirmala, M.; Viswanathamurthi, P. Design and synthesis of ruthenium (II) OCO pincer type NHC complexes and their catalytic role towards the synthesis of amides. J. Chem. Sci. 2016, 128, 9–21. [Google Scholar] [CrossRef]
- Selvamurugan, S.; Ramachandran, R.; Prakash, G.; Viswanathamurthi, P.; Malecki, J.G.; Endo, A. Ruthenium (II) carbonyl complexes containing bidentate 2-oxo-1,2-dihydroquinoline-3-carbaldehyde hydrazone ligands as efficient catalysts for catalytic amidation reaction. J. Organomet. Chem. 2016, 803, 119–127. [Google Scholar] [CrossRef]
- Selvamurugan, S.; Ramachandran, R.; Prakash, G.; Nirmala, M.; Viswanathamurthi, P.; Fujiwara, S.; Endo, A. Ruthenium (II) complexes encompassing 2-oxo-1,2-dihydroquinoline-3-carbaldehyde thiosemicarbazone hybrid ligand: A new versatile potential catalyst for dehydrogenative amide synthesis. Inorg. Chim. Acta 2017, 454, 46–53. [Google Scholar] [CrossRef]
- Higuchi, T.; Tagawa, R.; Iimuro, A.; Akiyama, S.; Nagae, H.; Mashima, K. Tunable ligand effects on ruthenium catalyst activity for selectively preparing imines or amides by dehydrogenative coupling reactions of alcohols and amines. Chem. Eur. J. 2017, 23, 12795–12804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, M.Q.; Cheng, C.X.; Wang, H.J.; Lu, Q.; Liu, H.F.; Yao, F.B.; Chen, C.; Verpoort, F. In situ generated ruthenium catalyst systems bearing diverse N-heterocyclic carbene precursors for atom-economic amide synthesis from alcohols and amines. Chem. Asian J. 2018, 13, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, M.Q.; Zhang, N.; Wang, H.J.; Miao, Y.; Su, W.; Yuan, Y.; Chen, C.; Verpoort, F. Efficient N-heterocyclic carbene/ruthenium catalytic systems for the alcohol amidation with amines: Involvement of poly-carbene complexes? ChemCatChem 2018. [Google Scholar] [CrossRef]
- Maji, M.; Chakrabarti, K.; Paul, B.; Roy, B.C.; Kundu, S. Ruthenium(II)-NNN-pincer-complex-catalyzed reactions between various alcohols and amines for sustainable C-N and C-C bond formation. Adv. Synth. Catal. 2018, 360, 722–729. [Google Scholar] [CrossRef]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C-NMR spectroscopic determination of ligand donor strengths using N-heterocyclic carbene complexes of palladium (II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Chen, C.; Kim, M.H.; Hong, S.H. N-heterocyclic carbene-based ruthenium-catalyzed direct amidation of aldehydes with amines. Org. Chem. Front. 2015, 2, 241–247. [Google Scholar] [CrossRef]
- Kaufhold, S.; Petermann, L.; Staehle, R.; Rau, S. Transition metal complexes with N-heterocyclic carbene ligands: From organometallic hydrogenation reactions toward water splitting. Coord. Chem. Rev. 2015, 304, 73–87. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 3a–3t are available from the authors. |




| Entry | L | x | y | n | Yields (%) b | ||
|---|---|---|---|---|---|---|---|
| 3a | 4a | Unreacted 1a | |||||
| 1 | L1 | 2.00 | 3.50 | 0.5 | 62 | 15 | 18 |
| 2 | L2 | 2.00 | 3.50 | 0.5 | 28 | 30 | 39 |
| 3 | L3 | 2.00 | 3.50 | 0.5 | 63 | 15 | 16 |
| 4 | L4 | 2.00 | 3.50 | 0.5 | 78 | 10 | 8 |
| 5 | L5 | 2.00 | 3.50 | 0.5 | 72 | 12 | 8 |
| 6 | L6 | 2.00 | 3.50 | 0.5 | 28 | 30 | 39 |
| 7 | L4 | 2.00 | 3.50 | 0.0 | 57 | 10 | 31 |
| 8 | L4 | 2.00 | 3.50 | 1.0 | 79 | 6 | 8 |
| 9 | L4 | 2.00 | 3.50 | 1.5 | 81 | 5 | 7 |
| 10 | L4 | 2.00 | 3.50 | 2.0 | 83 | 4 | 5 |
| 11 | L4 | 2.00 | 3.50 | 2.5 | 82 | 4 | 6 |
| 12 | L4 | 0.00 | 1.50 | 2.0 | 0 | 19 | 76 |
| 13 | L4 | 0.50 | 2.00 | 2.0 | 37 | 10 | 51 |
| 14 | L4 | 1.00 | 2.50 | 2.0 | 60 | 11 | 28 |
| 15 | L4 | 1.50 | 3.00 | 2.0 | 75 | 7 | 16 |
| 16 | L4 | 2.50 | 4.00 | 2.0 | 86 | 4 | 9 |
| 17 | L4 | 3.00 | 4.50 | 2.0 | 81 | 6 | 3 |
| 18 c | L4 | 2.50 | 4.00 | 2.0 | 93 | 5 | 0 |

| Entry | Base | x | y | Yields (%) b | ||
|---|---|---|---|---|---|---|
| 3a | 4a | Unreacted 1a | ||||
| 1 | NaH | 2.00 | 1.50 | 65 | 7 | 24 |
| 2 | KHMDS | 2.00 | 1.50 | 27 | 11 | 57 |
| 3 | KOtBu | 2.00 | 1.50 | 45 | 15 | 32 |
| 4 | Cs2CO3 | 2.00 | 1.50 | 86 | 7 | 5 |
| 5 | Cs2CO3 | 2.00 | 0.50 | 57 | 18 | 22 |
| 6 | Cs2CO3 | 2.00 | 1.00 | 71 | 13 | 12 |
| 7 | Cs2CO3 | 2.00 | 2.00 | 69 | 16 | 13 |
| 8 | Cs2CO3 | 2.00 | 2.50 | 45 | 38 | 15 |
| 9 | Cs2CO3 | 1.50 | 1.50 | 66 | 15 | 12 |
| 10 | Cs2CO3 | 1.75 | 1.50 | 90 | 7 | 2 |
| 11 | Cs2CO3 | 2.25 | 1.50 | 81 | 10 | 8 |
| 12 | Cs2CO3 | 2.50 | 1.50 | 72 | 12 | 15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Miao, Y.; De Winter, K.; Wang, H.-J.; Demeyere, P.; Yuan, Y.; Verpoort, F. Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines. Molecules 2018, 23, 2413. https://doi.org/10.3390/molecules23102413
Chen C, Miao Y, De Winter K, Wang H-J, Demeyere P, Yuan Y, Verpoort F. Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines. Molecules. 2018; 23(10):2413. https://doi.org/10.3390/molecules23102413
Chicago/Turabian StyleChen, Cheng, Yang Miao, Kimmy De Winter, Hua-Jing Wang, Patrick Demeyere, Ye Yuan, and Francis Verpoort. 2018. "Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines" Molecules 23, no. 10: 2413. https://doi.org/10.3390/molecules23102413
APA StyleChen, C., Miao, Y., De Winter, K., Wang, H. -J., Demeyere, P., Yuan, Y., & Verpoort, F. (2018). Ruthenium-Based Catalytic Systems Incorporating a Labile Cyclooctadiene Ligand with N-Heterocyclic Carbene Precursors for the Atom-Economic Alcohol Amidation Using Amines. Molecules, 23(10), 2413. https://doi.org/10.3390/molecules23102413