Near Infrared Fluorophore-Tagged Chloroquine in Plasmodium falciparum Diagnostic Imaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Squaraine NIR Dye Conjugated with Chloroquine
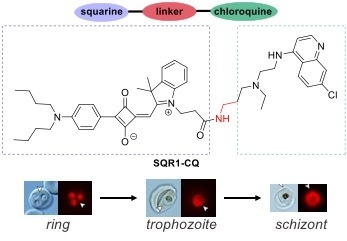
2.2. Labelling Applications of SQR1-CQ on P. falciparum
2.3. Comparison of SQR1-CQ with Commercial CQ-BODIPY
2.4. IC50 Studies
2.5. Fluorescence Staining on Unfixed Thin Blood Smears
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of Compounds
3.3. Preparation of Labelling Reagents
3.4. Parasite Culture and Synchronization
3.5. Assessment of Parasite Fluorescence Labelling via Confocal Imaging
3.6. Fluorescence Labelling of Unfixed Thin Smear Preparations
3.7. P. falciparum Erythrocyte Invasion Half-Maximal Inhibitory Concentration (IC50)
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loh, C.C.Y.; Suwanarusk, R.; Lee, Y.Q.; Chan, K.W.K.; Choy, K.-Y.; Rénia, L.; Russell, B.; Lear, M.J.; Nosten, F.H.; Tan, K.S.W.; et al. Characterization of the commercially-available fluorescent chloroquine-BODIPY conjugate, LynxTag-CQGREEN, as a marker for chloroquine resistance and uptake in a 96-Well plate assay. PLoS ONE 2014, 9, e110800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimi, M.; Sankar, V.; Rahim, M.K.A.; Nitha, P.R.; Das, S.; Radhakrishnan, K.V.; Raghu, K.G. Novel glycoconjugated squaraine dyes for selective optical imaging of cancer cells. Chem. Commun. 2017, 53, 5433–5436. [Google Scholar] [CrossRef] [PubMed]
- Slater, A. Chloroquine: Mechanism of drug action and resistance in plasmodium falciparum. Pharmacol. Ther. 1993, 57, 203–235. [Google Scholar] [CrossRef]
- Homewood, C.A.; Warhurst, D.C.; Peters, W.; Baggaley, V.C. Lysosomes, pH and the anti-malarial action of chloroquine. Nature 1972, 235, 50–52. [Google Scholar] [CrossRef] [PubMed]
- de Duve, C.; de Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Van Schalkwyk, D.A.; Saliba, K.J.; Biagini, G.A.; Bray, P.G.; Kirk, K. Loss of pH Control in Plasmodium falciparum Parasites Subjected to Oxidative Stress. PLoS ONE 2013, 8, e58933. [Google Scholar] [CrossRef] [PubMed]
- Saliba, K.J.; Kirk, K. pH Regulation in the intracellular malaria parasite. J. Biol. Chem. Available online: http://www.jbc.org/content/274/47/33213.long (accessed on 26 August 2018).
- Pleijhuis, R.G.; Langhout, G.C.; Helfrich, W.; Themelis, G.; Sarantopoulos, A.; Crane, L.M.; Harlaar, N.J.; de Jong, J.S.; Ntziachristos, V.; van Dam, G.M. Near-infrared fluorescence (NIRF) imaging in breast conserving surgery: Assessing intraoperative techniques in tissue simulating breast phantoms. Eur. J. Surg. Oncol. 2011, 37, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Pauli, J.; Vag, T.; Haag, R.; Spieles, M.; Wenzel, M.; Kaiser, W.A.; Resch-Genger, U.; Hilger, I. An in vitro characterization study of new near infrared dyes for molecular imaging. Eur. J. Med. Chem. 2009, 44, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Tromberg, B.J.; Pogue, B.W.; Paulsen, K.D.; Yodh, A.G.; Boas, D.A.; Cerussi, A.E. Assessing the future of diffuse optical imaging technologies for breast cancer management. Med. Phys. 2008, 35, 2443–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Burstin, J.; Eser, S.; Seidler, B.; Meining, A.; Bajbouj, M.; Mages, J.; Lang, R.; Kind, A.J.; Schnieke, A.E.; Schmid, R.M.; et al. Highly sensitive detection of early-stage pancreatic cancer by multimodal nearinfrared molecular imaging in living mice. Int. J. Cancer 2008, 123, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, N.; Yamazaki, R.; Nakagawa, A.; Abe, A.; Hamada, K.; Kubota, K.; Oyama, T. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am. J. Surg. 2008, 195, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Eck, P.K.; Baidoo, K.E.; Choyke, P.L.; Brechbiel, M.W. Toward preparation of antibody-based imaging probe libraries for dual-modality positron emission tomography and fluorescence imaging. Bioorg. Med. Chem. 2009, 17, 5176–5181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobedo, J.O.; Rusin, O.; Lim, S.; Strongin, R.M. NIR dyes for bioimaging applications. Curr. Opin. Chem. Biol. 2010, 14, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Yang, D.; Wang, Q.; Yang, L.; Sasabe, H.; Sano, T.; Kido, J.; Lu, Z.; Huang, Y. Central dicyanomethylene-substituted unsymmetrical squaraines and their application in organic solar cells. J. Mater. Chem. A 2018, 6, 5797–5806. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular imaging: Current status and emerging strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cho, B.; Chan, L.Y.; Kwan, W.L.; Lee, C.L.K. Development of asymmetrical near infrared squaraines with large Stokes shift. RSC Adv. 2015, 5, 106868–106876. [Google Scholar] [CrossRef]
- Li, F.; Gao, N.; Xu, H.; Liu, W.; Shang, H.; Yang, W.; Zhang, M. Relationship between molecular stacking and optical properties of 9,10-bis((4-N,N-dialkylamino)styryl) anthracene crystals: The cooperation of excitonic and dipolar coupling. Chem. Eur. J. 2014, 20, 9991–9997. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, E.; Chithra, P.; Ajayaghosh, A. A Controlled supramolecular approach toward cation-specific chemosensors: Alkaline earth metal ion-driven exciton signaling in squaraine tethered podands. J. Am. Chem. Soc. 2004, 126, 6590–6598. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, W.; Sun, K.; Deng, K.; Zhang, W.; Wang, Z.; Jiang, X. Resettable, multi–readout logic gates based on controllably reversible aggregation of gold nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 4103–4107. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.H.; Wu, W.J.; Hua, J.L.; Jing, Y.H.; Meng, F.S.; Tian, H. Photovoltaic properties of new cyanine–naphthalimide dyads synthesized by ‘Click’ chemistry. Tetrahedron Lett. 2007, 48, 2461–2465. [Google Scholar] [CrossRef]
- Dong, S.; Teo, J.D.W.; Chan, L.Y.; Lee, C.L.K.; Sou, K. Far-red fluorescent liposomes for folate receptor-targeted bioimaging. ACS Appl. Nano Mater. 2018, 1, 1009–1013. [Google Scholar] [CrossRef]
- Boudhar, A.; Ng, X.W.; Loh, C.Y.; Chia, W.N.; Tan, Z.M.; Nosten, F.; Dymock, B.W.; Tan, K.S.W. Overcoming chloroquine resistance in malaria: Design, synthesis and structure-activity relationships of novel hybrid compounds. Antimicrob. Agents Chemother. 2016, 60, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, A.; Pradipta, A.R.; Kitazume, S.; Taniguchic, N.; Tanaka, K. Effect of spermine-derived AGEs on oxidative stress and polyamine metabolism. Org. Biomol. Chem. 2017, 15, 6720–6724. [Google Scholar] [CrossRef] [PubMed]
- Kiakos, K.; Englinger, B.; Yanow, S.K.; Wernitznig, D.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Hartley, J.A.; Lee, M.; Patil, P.C. Design, synthesis, nuclear localization and biological activity of a fluorescent duocarmycin analog, HxTfA. Bioorg. Med. Chem. Lett. 2018, 28, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Ikegami-Kawai, M.; Arai, C.; Ogawa, Y.; Yanoshita, R.; Ihara, M. Selective accumulation of a novel antimalarial rhodacyanine derivative, SSJ-127, in an organelle of Plasmodium berghei. Bioorg. Med. Chem. 2010, 18, 7804–7808. [Google Scholar] [CrossRef] [PubMed]
- WHO Basic Malaria Microscopy—Part I: Learner’s Guide, 2nd ed. Available online: http://www.who.int/malaria/publications/atoz/9241547820/en/ (accessed on 12 August 2018).
- Wunderlich, J.; Rohrbach, P.; Dalton, J.P. The malaria digestive vacuole. Front. Biosci. (Sch. Ed.) 2012, 4, 1424–1448. [Google Scholar] [PubMed]
- Yayon, A.; Cabantchik, Z.I.; Ginsburg, H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984, 3, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Collot, M.; Kreder, R.; Tatarets, A.L.; Patsenker, L.D.; Mely, Y.; Klymchenko, A.S. Bright fluorogenic squaraines with tuned cell entry for selective imaging of plasma membrane vs. endoplasmic reticulum. Chem. Commun. 2015, 51, 17136–17139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, A.; Tiffert, T.; Mauritz, J.M.; Schlachter, S.; Bannister, L.H.; Kaminski, C.F.; Lew, V.L. FRET imaging of hemoglobin concentration in Plasmodium falciparum-infected red cells. PLoS ONE 2008, 3, e3780. [Google Scholar] [CrossRef] [PubMed]
- Horecker, B.L. The absorption spectra of hemoglobin and its derivatives in the visible and near infra-red regions. J. Biol. Chem. 1943, 148, 173–183. [Google Scholar]
- Abshire, J.R.; Rowlands, C.J.; Ganesan, S.M.; So, P.T.C.; Niles, J.C. Quantification of labile heme in live malaria parasites using a genetically encoded biosensor. Proc. Natl. Acad. Sci. USA 2017, 114, E2068–E2076. [Google Scholar] [CrossRef] [PubMed]
- Moneriz, C.; Marín-García, P.; Bautista, J.M.; Diez, A.; Puyet, A. Haemoglobin interference and increased sensitivity of fluorimetric assays for quantification of low-parasitaemia Plasmodium infected erythrocytes. Malar. J. 2009, 8, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.R.; Fujioka, H.; Williams, P.S.; Chalmers, J.J.; Grimberg, B.; Zimmerman, P.; Zborowski, M. Hemoglobin degradation in malaria-infected erythrocytes determined from live cell magnetophoresis. FASEB J. 2006, 20, 747–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, S.N.; Nash, M.N.; Baker, E.S.; Webster, M.W.; Lehane, A.M.; Shafik, S.H.; Martin, R.E. Molecular Mechanisms for Drug Hypersensitivity Induced by the Malaria Parasite’s Chloroquine Resistance Transporter. PLoS Pathog. 2016, 12, e1005725. [Google Scholar] [CrossRef] [PubMed]
- Hathiwala, R.; Mehta, P.R.; Nataraj, G.; Hathiwala, S. LED fluorescence microscopy: Novel method for malaria diagnosis compared with routine methods. J. Infect. Public Health 2017, 10, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Lenz, D.; Kremsner, P.G.; Lell, B.; Biallas, B.; Boettcher, M.; Mordmüller, B.; Adegnika, A.A. Assessment of LED fluorescence microscopy for the diagnosis of Plasmodium falciparum infections in Gabon. Malar. J. 2011, 10, 194. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are all available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, L.Y.; Teo, J.D.W.; Tan, K.S.-W.; Sou, K.; Kwan, W.L.; Lee, C.-L.K. Near Infrared Fluorophore-Tagged Chloroquine in Plasmodium falciparum Diagnostic Imaging. Molecules 2018, 23, 2635. https://doi.org/10.3390/molecules23102635
Chan LY, Teo JDW, Tan KS-W, Sou K, Kwan WL, Lee C-LK. Near Infrared Fluorophore-Tagged Chloroquine in Plasmodium falciparum Diagnostic Imaging. Molecules. 2018; 23(10):2635. https://doi.org/10.3390/molecules23102635
Chicago/Turabian StyleChan, Li Yan, Joshua Ding Wei Teo, Kevin Shyong-Wei Tan, Keitaro Sou, Wei Lek Kwan, and Chi-Lik Ken Lee. 2018. "Near Infrared Fluorophore-Tagged Chloroquine in Plasmodium falciparum Diagnostic Imaging" Molecules 23, no. 10: 2635. https://doi.org/10.3390/molecules23102635
APA StyleChan, L. Y., Teo, J. D. W., Tan, K. S. -W., Sou, K., Kwan, W. L., & Lee, C. -L. K. (2018). Near Infrared Fluorophore-Tagged Chloroquine in Plasmodium falciparum Diagnostic Imaging. Molecules, 23(10), 2635. https://doi.org/10.3390/molecules23102635