Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging
Abstract
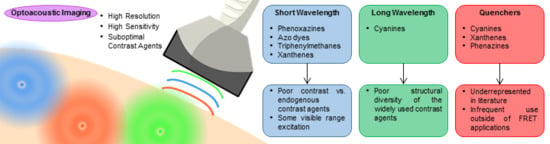
:1. Introduction
1.1. MSOT Introduction:
1.2. Exogenous Contrast for Optoacoustic Imaging
1.3. Near-Infrared Organic Dyes Introduction
2. Contrast Agents
2.1. Dyes with Absorbance < 720 nm
2.1.1. Evans Blue

2.1.2. Methylene Blue

2.1.3. Coomassie Blue

2.1.4. Rhodamine Dyes

2.2. Dyes with Absorbance > 720
2.2.1. Indocyanine Green

2.2.2. IR780 Iodide

2.2.3. IRDye800 CW

2.2.4. Alexa Fluor 750
2.3. Quenchers
2.3.1. IRDye QC-1

2.3.2. Black Hole Quencher 3 and QXL 680

2.3.3. QSY 21

3. Summary and Outlook
4. Materials and Methods for Optoacoustic Spectra in Figure 16
Author Contributions
Funding
Conflicts of Interest
References
- McNally, L.R.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.E.; Grizzle, W.E. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin. Cancer Res. 2016, 22, 3432–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliolanis, N.; Lasser, T.; Hyde, D.; Soubret, A.; Ripoll, J.; Ntziachristos, V. Free-space fluorescence molecular tomography utilizing 360° geometry projections. Opt. Lett. 2007, 32, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Schellenberger, E.A.; Ripoll, J.; Yessayan, D.; Graves, E.; Bogdanov, A.; Josephson, L.; Weissleder, R. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc. Natl. Acad. Sci. USA 2004, 101, 12294–12299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Udayakumar, T.S.; Wang, Z.; Dogan, N.; Pollack, A.; Yang, Y. Optical molecular imaging-guided radiation therapy part 2: Integrated X-ray and fluorescence molecular tomography. Med. Phys. 2017, 44, 4795–4803. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.P.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Lyu, Y.; Pu, K. Semiconducting oligomer nanoparticles as an activatable photoacoustic probe with amplified brightness for in vivo imaging of pH. Adv. Mater. 2016, 28, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, X.; Chen, J.; Zheng, J.; Cheng, Z.; Liu, Z. A self-assembled albumin-based nanoprobe for in vivo ratiometric photoacoustic pH imaging. Adv. Mater. 2015, 27, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Seeger, M.; Jiang, Y.; Mishra, A.; Sigmund, F.; Stelzl, A.; Lauri, A.; Symvoulidis, P.; Rolbieski, H.; Preller, M.; et al. Calcium Sensor for Photoacoustic Imaging. J. Am. Chem. Soc. 2017, 140, 2718–2721. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, P.; Smaga, L.P.; Hoffman, R.A.; Chan, J. Photoacoustic probes for ratiometric imaging of copper(II). J. Am. Chem. Soc. 2015, 137, 15628–15631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yan, X.; Sun, X.; Shen, B.; Gambhir, S.S. Tumor molecular imaging with nanoparticles. Engineering 2016, 2, 132–140. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Reis, C.A.; Costa, E.C.; Correia, I.J. Gold-core silica shell nanoparticles application in imaging and therapy: A review. Microporous Mesoporous Mater. 2018, 270, 168–179. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Controlled Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Xie, C.; Pu, K. Development of semiconducting polymer nanoparticles for photoacoustic imaging. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cui, D.; Fang, Y.; Zhen, X.; Upputuri, P.K.; Pramanik, M.; Ding, D.; Pu, K. Amphiphilic semiconducting polymer as multifunctional nanocarrier for fluorescence/photoacoustic imaging guided chemo-photothermal therapy. Biomaterials 2017, 145, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Cheng, P.; Pu, K. Synthesis of PEGylated semiconducting polymer amphiphiles for molecular photoacoustic imaging and guided therapy. Chem. Eur. J. 2018, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, A.; Shcherbakova, D.M.; Xia, J.; Wang, L.V.; Verkhusha, V.V. Multicontrast photoacoustic in vivo imaging using near-infrared fluorescent proteins. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filonov, G.S.; Krumholz, A.; Xia, J.; Yao, J.; Wang, L.V.; Verkhusha, V.V. Deep-tissue photoacoustic tomography of a genetically encoded near-infrared fluorescent probe. Angew. Chem. Int. Ed. 2012, 51, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pu, K. Advanced photoacoustic imaging applications of near-infrared absorbing organic nanoparticles. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Pu, K. Emerging designs of activatable photoacoustic probes for molecular imaging. Bioconjugate Chem. 2016, 27, 2808–2823. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.J.; Chan, J. Development of photoacoustic probes for in vivo molecular imaging. Biochemistry 2018, 57, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H. Near-Infrared Absorbing Nonmetallic Nanomaterials as Photoacoustic Contrast Agents for Biomedical Imaging. In Handbook of Ultrasonics and Sonochemistry; Ashokkumar, M., Ed.; Springer Science + Business Media: Singapore, 2016. [Google Scholar]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast agents for photoacoustic and thermoacoustic imaging: A review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef] [PubMed]
- Tansi, F.L.; Rüger, R.; Rabenhold, M.; Steiniger, F.; Fahr, A.; Kaiser, W.A.; Hilger, I. Liposomal encapsulation of a near-infrared fluorophore enhances fluorescence quenching and reliable whole body optical imaging upon activation in vivo. Small 2013, 9, 3659–3669. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef] [PubMed]
- Frenette, M.; Hatamimoslehabadi, M.; Bellinger-Buckley, S.; Laoui, S.; La, J.; Bag, S.; Mallidi, S.; Hasan, T.; Bouma, B.; Yelleswarapu, C.; et al. Shining light on the dark side of imaging: Excited state absorption enhancement of a bis-styryl BODIPY photoacoustic contrast agent. J. Am. Chem. Soc. 2014, 136, 15853–15856. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Müllen, K.; Yin, M. Water-soluble perylenediimides: Design concepts and biological applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, W.; Wei, J.; Li, C.; Liang, X.J.; Yin, M. Terrylenediimide-based intrinsic theranostic nanomedicines with high photothermal conversion efficiencey for photoacoustic imaging-guided cancer therapy. ACS Nano 2017, 11, 3797–3805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Wei, J.; Yin, M. Perylenediimide chromophore as an efficient photothermal agent for cancer therapy. Sci. Bull. 2018, 63, 101–107. [Google Scholar] [CrossRef]
- Flanagan, J.H.; Khan, S.H.; Menchen, S.; Soper, S.A.; Hammer, R.P. Functionalized tricarbocyanine dyes as near-infrared fluorescent probes for biomolecules. Bioconjugate Chem. 1997, 8, 751–756. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Patonay, G. Fluorescence studies of carbocyanines using AOTF. Talanta 1997, 45, 285–289. [Google Scholar] [CrossRef]
- Patonay, G.; Salon, J.; Sowell, J.; Strekowski, L. Noncovalent labeling of biomolecules with red and near-infrared dyes. Molecules 2004, 9, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, G.; Yang, V.W.; Cheng, T.; et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Nasr, K.; Alyabyev, S.; Feith, D.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Hyun, H.; Patonay, G.; Strekowski, L.; et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew. Chem. Int. Ed. Engl. 2011, 50, 6258–6263. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Henary, M.; Gao, T.; Narayana, L.; Owens, E.A.; Lee, J.H.; Park, G.; Wada, H.; Ashitate, Y.; Frangioni, G.V.; et al. 700-nm Zwitterionic Near-Infrared Fluorophores for Dual-Channel Image-Guided Surgery. Mol. Imaging Biol. 2016, 18, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Njiojob, C.N.; Owens, E.A.; Narayana, L.; Hyun, H.; Choi, H.S.; Henary, M. Tailored near-infrared contrast agents for image guided surgery. J. Med. Chem. 2015, 58, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.P.; Han, H.H.; Lu, J.; Yang, P.P.; Gao, Y.J.; An, H.W.; Zhanng, D.; Li, L.Z.; Zhang, J.P.; Wang, D.; et al. Charge transfer NIR dyes for improved photoacoustic effect. Dyes Pigments 2016, 125, 392–398. [Google Scholar] [CrossRef]
- Laufer, J.; Zhang, E.; Beard, P. Evaluation of absorbing chromophores used in tissue phantoms for quantitative photoacoustic spectroscopy and imaging. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 600–607. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Zeng, Z.; Xie, W.; Chen, W.R. Determination of optical absorption coefficient with focusing photoacoustic imaging. J. Biomed. Opt. 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.K.; Zhang, C.; Maslov, K.; Wang, L.V. Photoacoustic measurement of the Gruneisen parameter of tissue. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.B. The contribution of I 131-labeled proteins to measurements of blood volume. Ann. N. Y. Acad. Sci. 1957, 70, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Manaenko, A.; Chen, H.; Kammer, J.; Zhang, J.H.; Tang, J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J. Neurosci. Methods 2011, 195, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Le, V.H.; Fishman, W.H. Combination of Evans blue with plasma protein; its significance in capillary permeability studies, blood dye disappearance curves, and its use as a protein tag. Am. J. Physiol. 1947, 151, 26–33. [Google Scholar]
- Patterson, C.E.; Rhoades, R.A.; Garcia, J.G. Evans blue dye as a marker of albumin clearance in cultured endothelial monolayer and isolated lung. J. Appl. Physiol. 1992, 72, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Maslov, K.; Hu, S.; Wang, L.V. Evans blue dye-enhanced capillary-resolution photoacoustic microscopy in vivo. J. Biomed. Opt. 2009, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Aguirre, A.; Gamelin, J.; Maurudis, A.; Zhu, Q.; Wang, L.V. Real-time photoacoustic tomography of cortical hemodynamics in small animals. J. Biomed. Opt. 2010, 15. [Google Scholar] [CrossRef] [PubMed]
- Gahan, P.B. Vital Dyes. In Plant Histochemistry and Cytochemistry: An Introduction; Academic Press: London, UK, 1984; pp. 125–126. [Google Scholar]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you—Methylene blue. Neurobiol. Aging 2011, 32, 7–16. [Google Scholar] [CrossRef] [PubMed]
- DiSanto, A.R.; Wagner, J.G. Pharmacokinetics of highly ionized drugs. II. Methylene blue—Absorption, metabolism, and excretion in man and dog after oral administration. J. Pharm. Sci. 1972, 61, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Morgounova, E.; Shao, Q.; Hackel, B.J.; Thomas, D.D.; Ashkenazi, S. Photoacoustic lifetime contrast between methylene blue monomers and self-quenched dimers as a model for dual-labeled activatable probes. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Stein, E.W.; Margenthaler, J.A.; Wang, L.V. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13. [Google Scholar] [CrossRef] [PubMed]
- Erpelding, T.N.; Kim, C.; Pramanik, M.; Jankovic, L.; Maslov, K.; Guo, Z.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Sentinel lymph nodes in the rat: Noninvasive photoacoustic and US imaging with a clinical US system. Radiology 2010, 256, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Uribe, A.; Erpelding, T.N.; Krumholz, A.; Ke, H.; Maslov, K.; Appleton, C.; Margenthaler, J.A.; Wang, L.V. Dual-Modality Photoacoustic and Ultrasound Imaging System for Noninvasive Sentinel Lymph Node Detection in Patients with Breast Cancer. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Song, W.; Huynh, E.; Kim, J.; Kim, J.; Helfield, B.L.; Leung, B.Y.; Goertz, D.E.; Zheng, G.; Oh, J.; et al. Methylene blue microbubbles as a model dual-modality contrast agent for ultrasound and activatable photoacoustic imaging. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Morgounova, E.; Jiang, C.; Choi, J.; Bischof, J.; Ashkenazi, S. In vivo photoacoustic lifetime imaging of tumor hypoxia in small animals. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Y.; Chen, W.; Xu, Q.; Liu, Z. Hybrid polymeric nanoprobes for folate receptor-targeted photoacoustic imaging in vivo. Mater. Chem. Front. 2017, 1, 916–921. [Google Scholar] [CrossRef]
- Kelner, M.J.; Bagnell, R.; Hale, B.; Alexander, N.M. Methylene blue competes with paraquat for reduction by flavo-enzymes resulting in decreased superoxide production in the presence of heme proteins. Arch. Biochem. Biophys. 1988, 262, 422–426. [Google Scholar] [CrossRef]
- Butt, R.H.; Coorssen, J.R. Coomassie blue as a near-infrared fluorescent stain: A systematic comparison with Sypro Ruby for in-gel protein detection. Mol. Cell Proteomics 2013, 12, 3834–3850. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Wang, X.; Lee, Y.K.; Hah, H.J.; Kim, G.; Chen, T.; Orringer, D.A.; Sagher, O.; Liu, X.; Kopelman, R. Targeted blue nanoparticles as photoacoustic contrast agent for brain tumor delineation. Nano Res. 2011, 4, 1163–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orringer, D.; Sagher, O.; Kopelman, R.; Lee, Y.E.K. Dye-Loaded Nanoparticle. U.S. Patent 2010098637 (A1), 22 April 2010. [Google Scholar]
- Sakanoue, J.; Ichikawa, K.; Nomura, Y.; Tamura, M. Rhodamine 800 as a probe of energization of cells and tissues in the near-infrared region: A study with isolated rat liver mitochondria and hepatocytes. J. Biochem. 1997, 121, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.M.; Stephens, S. Flow cytometry of DNA content using oxazine 750 or related laser dyes with 633 nm excitation. Cytometry 1986, 7, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, S.V.; Huang, J.S.; Yin, W.; Albeituni, S.; Rush, J.; Khanal, A.; Yan, J.; Ceresa, B.P.; Frieboes, H.B.; McNally, L.R. Targeted noninvasive imaging of EGFR-expressing orthotopic pancreatic cancer using multispectral optoacoustic tomography. Cancer Res. 2014, 74, 6271–6279. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimbrough, C.W.; Hudson, S.; Khanal, A.; Egger, M.E.; McNally, R.L. Orthotopic pancreatic tumors detected by optoacoustic tomography using Syndecan-1. J. Surg. Res. 2015, 193, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, M.; Han, K.-T.; Müller, R.; Nord, S.; Schulz, A.; Seeger, S.; Wolfrum, J.; Arden-Jacob, J.; Deltau, G.; Marx, N.J.; et al. New fluorescent dyes in the red region for biodiagnostics. J. Fluoresc. 1995, 5, 247–261. [Google Scholar] [CrossRef] [PubMed]
- FDA Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=011525 (accessed on 21 November 2017).
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- NCI Drug Dictionary Entry for ICG. Indocyanine Green Solution. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug?cdrid=540122 (accessed on 21 November 2017).
- Buehler, A.; Herzog, E.; Razansky, D.; Ntzchristos, V. Video rate optoacoustic tomography of mouse kidney perfusion. Opt. Lett. 2010, 35, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Rajian, J.R.; Fabilli, M.L.; Fowlkes, J.B.; Carson, P.L.; Wang, X. Drug delivery monitoring by photoacoustic tomography with an ICG encapsulated double emulsion. Opt. Express. 2011, 19, 14335–14347. [Google Scholar] [CrossRef] [PubMed]
- Davies-Venn, C.A.; Angermiller, B.; Wilganowski, N.; Ghosh, P.; Harvey, B.R.; Wu, G.; Kwoon, S.; Aldrich, M.B.; Sevick-Muraca, E.M. Albumin-binding domain conjugate for near-infrared fluorescence lymphatic imaging. Mol. Imaging Biol. 2012, 14, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Morscher, S.; Driessen, W.H.; Claussen, J.; Burton, N.C. Semi-quantitative Multispectral Optoacoustic Tomography (MSOT) for volumetric PK imaging of gastric emptying. Photoacoustics 2014, 2, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Haritoglou, C.; Gandorfer, A.; Schaumberger, M.; Tadayoni, R.; Gandorfer, A.; Kampik, A. Light-absorbing properties and osmolarity of indocyanine-green depending on concentration and solvent medium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2722–2729. [Google Scholar] [CrossRef]
- Shigeta, Y.; Agano, T.; Sato, N.; Nakatsuka, H.; Kitagawa, K.; Hanaoka, T.; Morisono, K.; Tanaka, C. Detection of ICG at low concentrations by photoacoustic imaging system using LED light source. In Photons Plus Ultrasound: Imaging and Sensing 2017; Proc. SPIE: San Francisco, CA, USA, 3 March 2017. [Google Scholar]
- Yi, X.; Zhang, J.; Yan, F.; Lu, Z.; Huang, J.; Pan, C.; Yuan, J.; Zheng, W.; Zhang, K.; Wei, D.; et al. Synthesis of IR-780 dye-conjugated abiraterone for prostate cancer imaging and therapy. Int. J. Oncol. 2016, 49, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Jiang, Y.; Roberts, S.; Ntziachristos, V.; Westmeyer, G.G. Near-infrared photoacoustic imaging probe responsive to calcium. Anal. Chem. 2016, 88, 10785–10789. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Kimbrough, C.W.; Burton, N.C.; Morscher, S.; Egger, M.; McMasters, K.; Woloszynska-Read, A.; El-Baz, A.; McNally, L.R. Detection of microspheres in vivo using multispectral optoacoustic tomography. Biotech. Histochem. 2017, 92, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiderman, M.R.; Morgan, D.E.; Christein, J.D.; Grizzle, W.E.; McMasters, K.E.; McNally, L.R. Acidic pH-targeted chitosan-capped mesoporous silica coated gold nanorods facilitate detection of pancreatic tumors via multispectral optoacoustic tomography. ACS Biomater. Sci. Eng. 2016, 2, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Gerling, M.; Zhao, Y.; Nania, S.; Norberg, K.J.; Verbeke, C.S.; Englert, B.; Kuiper, R.V.; Bergström, A.; Hassan, M.; Neesse, A.; et al. Real-time assessment of tissue hypoxia in vivo with combined photoacoustics and high-frequency ultrasound. Theranostics 2014, 4, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Draney, D.; Sevick-Muraca, E.M.; Olive, D.M. Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats. Mol. Imaging Biol. 2010, 12, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Chekkoury, A.; Gateau, J.; Driessen, W.; Symvoulidis, P.; Bézière, N.; Feuchtinger, A.; Walch, A.; Ntziachristos, V. Optical mesoscopy without the scatter: Broadband multispectral optoacoustic mesoscopy. Biomed. Opt. Express. 2015, 6, 3134–3148. [Google Scholar] [CrossRef] [PubMed]
- Mercep, E.; Burton, N.C.; Claussen, J.; Razansky, D. Whole-body live mouse imaging by hybrid reflection-mode ultrasound and optoacoustic tomography. Opt. Lett. 2015, 40, 4643–4646. [Google Scholar] [CrossRef] [PubMed]
- Scarfe, L.; Rak-Raszewska, A.; Geraci, S.; Darssan, D.; Sharkey, J.; Huang, J.; Burton, N.C.; Mason, D.; Ranjzad, P.; Kenny, S.; et al. Measures of kidney function by minimally invasive techniques correlate with histological glomerular damage in SCID mice with adriamycin-induced nephropathy. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Stammes, M.A.; van Driel, P.B.; Crruz, L.J.; Knol-Blankevoort, V.T.; Löwik, M.A.; Mezzanotte, L.; Que, I.; Chan, A.; van den Wijngaard, J.P.; et al. Necrosis avid near infrared fluorescent cyanines for imaging cell death and their use to monitor therapeutic efficacy in mouse tumor models. Oncotarget 2015, 6, 39036–39049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederick, P.J.; Green, H.N.; Huang, J.S.; Egger, M.E.; Frieboes, H.B.; Grzzle, W.E.; McNally, L.R. Chemoresistance in ovarian cancer linked to expression of microRNAs. Biotech. Histochem. 2013, 88, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Wang, S.; Reinecke, D.; Kiser, W., Jr.; Kruger, R.A.; Degrado, T.R. Synthesis and evaluation of near-infrared (NIR) dye-herceptin conjugates as photoacoustic computed tomography (PCT) probes for HER2 expression in breast cancer. Bioconjugate Chem. 2008, 19, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Levi, J.; Kothapalli, S.R.; Ma, T.J.; Hartman, K.; Khuri-Yakub, B.T.; Gambhir, S.S. Design, synthesis, and imaging of an activatable photoacoustic probe. J. Am. Chem. Soc. 2010, 132, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Larina, I.V.; Larin, K.V.; Esenaliev, R.O. Real-time optoacoustic monitoring of temperature in tissues. J. Phys. D-Appl. Phys. 2005, 38, 2633–2639. [Google Scholar] [CrossRef]
- Johansson, M.K.; Fidder, H.; Dick, D.; Cook, R.M. Intramolecular dimers: A new strategy to fluorescence quenching in dual-labeled oligonucleotide probes. J. Am. Chem. Soc. 2002, 124, 6950–6956. [Google Scholar] [CrossRef] [PubMed]
- Matayoshi, E.D.; Wang, G.T.; Krafft, G.A.; Erickson, J. Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science 1990, 247, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.C.; Tucker, R.A.; Holloway, B.P.; Icenogle, J.P. A sensitive, type-specific, fluorogenic probe assay for detection of human papillomavirus DNA. J. Clin. Microbiol. 1997, 35, 886–891. [Google Scholar] [PubMed]
- Peng, X.; Chen, H.; Draney, D.R.; Volcheck, W.; Schutz-Geschwender, A.; Olive, D.M. A nonfluorescent, broad-range quencher dye for Forster resonance energy transfer assays. Anal. Biochem. 2009, 388, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Marras, S.A.E.; Kramer, F.R.; Tyagi, S. Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes. Nucleic. Acids Res. 2002, 30, e122. [Google Scholar] [CrossRef] [PubMed]
- De la Zerda, A.; Bodapati, S.; Teed, R.; May, S.Y.; Tabakman, S.M.; Liu, Z.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano 2012, 6, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Hoon, H.; Tawney, J.G.; Choi, H.S.; Henary, M. Correlating molecular character of NIR imaging agents with tissue-specific uptake. J. Med. Chem. 2015, 58, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Rettig, W.; Fiedeldei, U.; Baumgärtel, H. Non-radiative deactivation via biradicaloid charge-transfer states in oxazine and thiazine dyes. Chem. Phys. Lett. 1988, 148, 347–352. [Google Scholar] [CrossRef]
- Pansare, V.; Hejazi, S.; Faenza, W.; Prud’homme, R.K. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores and Multifunctional Nano Carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Samykutty, A.; Grizzle, W.E.; Fouts, B.L.; McNally, M.W.; Chuong, P.; Thomas, A.; Chiba, A.; Otali, D.; Woloszynska, A.; Said, N.; et al. Optoacoustic imaging identifies ovarian cancer using a microenvironment targeted theranostic wormhole mesoporous silica nanoparticle. Biomaterials 2018, 182, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.K.; Pender, D.; Chuong, P.; Fouts, B.L.; Sobelov, A.; McNally, M.W.; Mezera, M.; Woo, S.Y.; McNally, L.R. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J. Control Release 2016, 231, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
















| Molecular Weight | Rotatable Bonds | H-Bond Acceptors | Molar Absorptivity (M−1 cm−1) | Quantum Yield (%) | |
|---|---|---|---|---|---|
| DYES | |||||
| ICG | 751.97 | 10 | 6 | 183,000 | 3 |
| MB | 284.40 | 0 | 1 | 95,000 | 3 |
| Evans Blue | 868.85 | 4 | 20 | 7,810 | N/A |
| IR780 iodide | 540.20 | 4 | 0 | 274,000 | 7.6 |
| IRDye800CW | 1097.23 | 18 | 15 | 181,458 | 3.4 |
| Rhodamine 800 | 396.50 | 0 | 2 | 113,302 | 25 |
| Coomassie Blue | 802.98 | 11 | 7 | 43,000 | N/A |
| QUENCHERS | |||||
| IRDyeQC-1 | 1188.82 | 23 | 15 | 96,000 | N/A |
| BHQ3 | 535.66 | 7 | 7 | 42,700 | N/A |
| BlackBerry Quencher 650 | 576.60 | 6 | 12 | 41,000 | N/A |
| QSY21 | 779.88 | 4 | 5 | 90,000 | N/A |
| Molecular Volume (Å3) | LogD | Surface Area (Å2) | TPSA (Å2) | Polorizability | |
|---|---|---|---|---|---|
| DYES | |||||
| ICG | 686.93 | 5.53 | 1143.82 | 181.76 | 87.37 |
| MB | 261.02 | −0.62 | 388.00 | 43.91 | 32.74 |
| Evans Blue | 658.20 | −3.62 | 987.04 | 396.09 | 81.28 |
| IR780 iodide | 536.10 | 6.67 | 898.85 | 50.60 | 66.43 |
| IRDye800CW | 920.12 | −1.86 | 1536.72 | 385.83 | 108.94 |
| Rhodamine 800 | 363.29 | 1.09 | 584.65 | 87.53 | 48.92 |
| Coomassie Blue | 716.81 | 4.54 | 1167.33 | 216.29 | 93.26 |
| QUENCHERS | |||||
| IRDyeQC-1 | 1013.19 | −0.69 | 1698.30 | 379.84 | 120.15 |
| BHQ3 | 502.27 | 0.30 | 812.53 | 133.61 | 66.48 |
| BlackBerry Quencher 650 | 503.94 | 0.94 | 828.01 | 157.09 | 61.17 |
| QSY21 | 668.54 | 3.31 | 1055.44 | 173.18 | 89.10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laramie, M.D.; Smith, M.K.; Marmarchi, F.; McNally, L.R.; Henary, M. Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging. Molecules 2018, 23, 2766. https://doi.org/10.3390/molecules23112766
Laramie MD, Smith MK, Marmarchi F, McNally LR, Henary M. Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging. Molecules. 2018; 23(11):2766. https://doi.org/10.3390/molecules23112766
Chicago/Turabian StyleLaramie, Matt D., Mary K. Smith, Fahad Marmarchi, Lacey R. McNally, and Maged Henary. 2018. "Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging" Molecules 23, no. 11: 2766. https://doi.org/10.3390/molecules23112766
APA StyleLaramie, M. D., Smith, M. K., Marmarchi, F., McNally, L. R., & Henary, M. (2018). Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging. Molecules, 23(11), 2766. https://doi.org/10.3390/molecules23112766