Antiproliferative, Proapoptotic, Antioxidant and Antimicrobial Effects of Sinapis nigra L. and Sinapis alba L. Extracts
Abstract
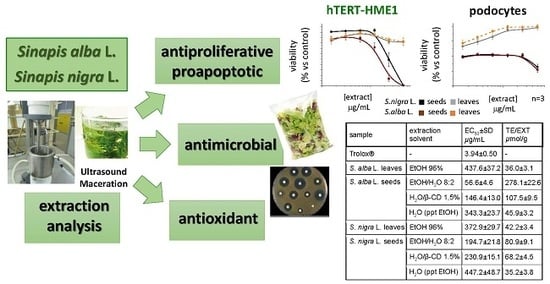
:1. Introduction
2. Results
2.1. Extract Preparation
2.2. Antioxidant Activity
2.3. Antiproliferative Activity
2.3.1. Effect on the Cell Viability of Non-Tumor and Tumor Cell Lines
2.3.2. Effects on MAPK
2.3.3. Effects on Cell Cycle
2.4. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Extraction Procedures
4.2.1. Method A
4.2.2. Method B
4.2.3. Method C
4.3. HPLC Analyses
4.4. Determination of Antioxidant Activity Using the DPPH• Radical Scavenging Method
4.5. Cell Culture
4.6. Western Blot Analysis
4.7. Cell Cycle
4.8. Antimicrobial Diffusion Test
4.9. Antimicrobial Activity in Salad
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Coz, C.J.; Ducombs, G. Plants and plant products. In Contact Dermatitis, 4th ed.; Frosch, P.J., Menne, T., Lepottevin, J.P., Eds.; Springer: Berlin/Heildeberg, Germany, 2006; pp. 751–800. [Google Scholar]
- Herr, I.; Buchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Li, F.; Lampe, J.W. Mechanisms of action of isothiocyanates in cancer chemoprevention: An update. Food Funct. 2011, 2, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, e1701000. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okunade, O.A.; Ghawi, S.K.; Methven, L.; Niranjan, K. Thermal and pressure stability of myrosinase enzymes from black mustard (Brassica nigra L. W.D.J. Koch. var. nigra), brown mustard (Brassica juncea L. Czern. var. juncea) and yellow mustard (Sinapsis alba L. subsp. maire) seeds. Food Chem. 2015, 187, 485–490. [Google Scholar] [CrossRef]
- Shishu, G.; Singla, A.K.; Kaur, I.P. Inhibition of mutagenicity of food-derived heterocyclic amines by sulphoraphene-an isothiocyanate isolated from radish. Planta Med. 2003, 69, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.A.; Haghi, A.; Rahmati, M.; Taniguchi, H.; Mocan, A.; Echeverria, J.; Gupta, V.K.; Tzvetkov, N.T.; Atanasov, A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018, 424, 46–69. [Google Scholar] [CrossRef] [PubMed]
- Herman-Antosiewicz, A.; Johnson, D.E.; Singh, S.V. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006, 66, 5828–5835. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, S.; Uehara, N.; Miki, H.; Yoshizawa, K.; Kawanaka, A.; Yuri, T.; Tsubura, A. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res. 2010, 30, 3381–3390. [Google Scholar] [PubMed]
- Nishikawa, T.; Tsuno, N.H.; Okaji, Y.; Shuno, Y.; Sasaki, K.; Hongo, K.; Sunami, E.; Kitayama, J.; Takahashi, K.; Nagawa, H. Inhibition of autophagy potentiates sulforaphane-induced apoptosis in human colon cancer cells. Ann. Surg. Oncol. 2010, 17, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Kalkunte, S.; Swamy, N.; Dizon, D.S.; Brard, L. Benzyl isothiocyanate (BITC) induces apoptosis in ovarian cancer cells in vitro. J. Exp. Ther. Oncol. 2006, 5, 287–300. [Google Scholar] [PubMed]
- Pal, S.; Konkimalla, V.B. Sulforaphane regulates phenotypic and functional switching of both induced and spontaneously differentiating human monocytes. Int. Immunopharmacol. 2016, 35, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.S.; Lee, S.Y.; Dougherty, R.H.; Kang, D.H. Antimicrobial effects of mustard flour and acetic acid against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2003, 69, 2959–2963. [Google Scholar] [CrossRef] [PubMed]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Holley, R.A. Control of Salmonella enterica and Listeria monocytogenes in hummus using allyl isothiocyanate. Inter. J. Food Microbiol. 2018, 278, 73–80. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the safety if allyl isothiocyanate for the proposed uses as food additive. EFSA J. 2010, 8, 1943. [Google Scholar]
- Tanase, C.; Cosarca, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. Antibacterial activities of beech bark (Fagus sylvatica L.) polyphenolic extract. Environ. Eng. Manag. J. 2018, 17, 877–884. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Normark, S.; Nilsson, C.; Normark, B.H.; Hornef, M.W. Persistent infection with Helicobacter pylori and the development of gastric cancer. Adv. Cancer Res. 2003, 90, 63–89. [Google Scholar] [PubMed]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simoes, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Toribio, A.; Nuzillard, J.M.; Pinel, B.; Boudesocque, L.; Lafosse, M.; De La Poype, F.; Renault, J.H. Pilot-scale ion-exchange centrifugal partition chromatography: Purification of sinalbin from white mustard seeds. J. Sep. Sci. 2009, 32, 1801–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantegna, S.; Binello, A.; Boffa, L.; Giorgis, M.; Cena, C.; Cravotto, G. A one-pot ultrasound-assisted water extraction/cyclodextrin encapsulation of resveratrol from Polygonum cuspidatum. Food Chem. 2012, 130, 746–750. [Google Scholar] [CrossRef]
- Boffa, L.; Binello, A.; Boscaro, V.; Gallicchio, M.; Amisano, G.; Fornasero, S.; Cravotto, G. Commiphora myrrha (Nees) Engl. extracts: Evaluation of antioxidant and antiproliferative activity and their ability to reduce microbial growth on fresh-cut salad. Int. J. Food Sci. Techonol. 2016, 51, 625–632. [Google Scholar] [CrossRef]
- Tse, G.; Eslick, G.D. Cruciferous vegetables and risk of colorectal neoplasms: A systematic review and meta-analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, R.; Yu, Q.; Potter, J.; Chiba, M. Direct and simultaneous analysis of sinigrin and allyl isothiocyanate in mustard samples by high-performance liquid chromatography. J. Agric. Food Chem. 2002, 50, 4749–4753. [Google Scholar] [CrossRef] [PubMed]
- Borek, V.; Morra, M.J. Ionic thiocyanate (SCN-) production from 4-hydroxybenzyl glucosinolate contained in Sinapis alba seed meal. J. Agric. Food Chem. 2005, 53, 8650–8654. [Google Scholar] [CrossRef] [PubMed]
- Stoin, D.; Radu, F.; Dogaru, D. Researches regarding the isolation, purification and analysis of sinigrin glucosinolate from Brassica nigra and Armoracia rusticana. Bull. USAMV-CN 2007, 63, 77–82. [Google Scholar]
- Heaney, R.K.; Fenwich, G.R. Methods for glucosinolate analysis. In Methods in Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: London, UK, 1993; Volume 8, pp. 531–550. [Google Scholar]
- Herzallah, S.; Holley, R. Determination of sinigrin, sinalbin, allyl- and benzyl isothiocyanates by RP-HPLC in mustard powder extracts. LWT-Food Sci. Technol. 2012, 47, 293–299. [Google Scholar] [CrossRef]
- Aliakbarlu, J.; Mohammadi, S.; Khalili, S. A Study on Antioxidant Potency and Antibacterial Activity of Water Extracts of Some Spices Widely Consumed in Iranian Diet. J. Food Biochem. 2014, 38, 159–166. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Inter. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef] [PubMed]
- Plumb, G.W.; Lambert, N.; Chambers, S.J.; Wanigatunga, S.; Heaney, R.K.; Plumb, J.A.; Aruoma, O.I.; Halliwell, B.; Miller, N.J.; Williamson, G. Are whole extracts and purified glucosinolates from cruciferous vegetables antioxidants? Free Radic. Res. 1996, 25, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; An, H.; Feng, L.; Liu, Q.; Wang, S.; Zhang, T. Sinapine as an active compound for inhibiting the proliferation of Caco-2 cells via downregulation of P-glycoprotein. Food Chem. Toxicol. 2014, 67, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Dubie, J.; Stancik, A.; Morra, M.; Nindo, C. Antioxidant extraction from mustard (Brassica juncea) seed meal using high-intensity ultrasound. J. Food Sci. 2013, 78, E542–548. [Google Scholar] [CrossRef] [PubMed]
- Bhinu, V.S.; Schafer, U.A.; Li, R.; Huang, J.; Hannoufa, A. Targeted modulation of sinapine biosynthesis pathway for seed quality improvement in Brassica napus. Transgenic Res. 2009, 18, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Zhang, R.; Batra, S.; Shi, Y.; Srivastava, S.K. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis 2009, 30, 1744–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Su, Z.Z.; Lebedeva, I.V.; Sauane, M.; Gopalkrishnan, R.V.; Valerie, K.; Dent, P.; Fisher, P.B. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc. Natl. Acad. Sci. USA 2002, 99, 10054–10059. [Google Scholar] [CrossRef] [PubMed]
- Porras, A.; Zuluaga, S.; Black, E.; Valladares, A.; Alvarez, A.M.; Ambrosino, C.; Benito, M.; Nebreda, A.R. P38 alpha mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol. Biol. Cell 2004, 15, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, M.; Lu, Y.; Lu, Y.; Mills, G.B.; Fan, Z. Involvement of JNK-mediated pathway in EGF-mediated protection against paclitaxel-induced apoptosis in SiHa human cervical cancer cells. Br. J. Cancer 2001, 85, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Lara-Lledo, M.; Olaimat, A.; Holley, R.A. Inhibition of Listeria monocytogenes on bologna sausages by an antimicrobial film containing mustard extract or sinigrin. Int. J. Food Microbiol. 2012, 156, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Luciano, F.B.; Belland, J.; Holley, R.A. Microbial and chemical origins of the bactericidal activity of thermally treated yellow mustard powder toward Escherichia coli O157:H7 during dry sausage ripening. Int. J. Food Microbiol. 2011, 145, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Nilson, A.M.; Holley, R.A. Use of deodorized yellow mustard powder to control Escherichia coli O157:H7 in dry cured Westphalian ham. Food Microbiol. 2012, 30, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Sultanbawa, Y. Plants antimicrobias in food applications: Minireview. In Science against Microbial Pathogens: Communicating Current Research and Techonologial Adavances; Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2011; Volume 2, pp. 1084–1093. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.; Rinaldi, M.; Arlorio, M. Study of the DPPH•-scavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.C. Western Blot: Technique, Theory, and Trouble Shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef] [PubMed]




| S. alba Sample | Extraction Solvent (Plant/Solvent) | Extraction Method | Yield (w/w%) | GSB/EXT 1 (w/w%) | GSB/SAM 1 (mg/g) | SNP/EXT 1 (w/w%) | SNP/SAM 1 (mg/g) |
|---|---|---|---|---|---|---|---|
| Fresh leaves | EtOH 96% (1:7) | A | 3.68 | 2.54 | 0.93 | - | - |
| Seeds | EtOH/H2O 8:2 (1:10) | B | 12.5 | 11.5 | 14.4 | 6.96 | 8.75 |
| Seeds | H2O/β-CD 1.5% (1:10) | B | 11.9 | 0.30 | 0.35 | 0.20 | 0.24 |
| Seeds | H2O (ppt EtOH) (1:10) | C | 15.5 | 2.77 | 4.43 | 3.41 | 5.29 |
| S. nigra Sample | Extracted Solvent (Plant/Solvent) | Extraction Method | Yield (w/w%) | SNG/EXT 2 (w/w%) | SNG/SAM 2 (mg/g) | SNP/EXT 2 (w/w%) | SNP/SAM 2 (mg/g) |
| Freshleaves | EtOH 96% (1:7) | A | 3.43 | 1.86 | 0.64 | - | - |
| Seeds | EtOH/H2O 8:2 (1:10) | B | 11.1 | 17.7 | 19.7 | 4.5 | 5.1 |
| Seeds | H2O/β-CD 1.5% (1:10) | B | 10.1 | - | - | 0.32 | 0.32 |
| Seeds | H2O (ppt EtOH) (1:10) | C | 17.4 | 2.15 | 3.75 | 1.42 | 2.47 |
| Sample | Extraction Solvent | EC50 ± SD (μg/mL) | TE/EXT (μmol/g) |
|---|---|---|---|
| Trolox® | - | 3.94 ± 0.50 | - |
| S. alba L. leaves | EtOH 96% | 437.6 ± 37.2 | 36.0 ± 3.1 |
| S. alba L. seeds | EtOH/H2O 8:2 | 56.6 ± 4.6 | 278.1 ± 22.6 |
| H2O/β-CD 1.5% | 146.4 ± 13.0 | 107.5 ± 9.5 | |
| H2O (ppt EtOH) | 343.3 ± 23.7 | 45.9 ± 3.2 | |
| S. nigra L. leaves | EtOH 96% | 372.9 ± 29.7 | 42.2 ± 3.4 |
| S. nigra L. seeds | EtOH/H2O 8:2 | 194.7 ± 21.8 | 80.9 ± 9.1 |
| H2O/β-CD 1.5% | 230.9 ± 15.1 | 68.2 ± 4.5 | |
| H2O (ppt EtOH) | 447.2 ± 48.7 | 35.2 ± 3.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscaro, V.; Boffa, L.; Binello, A.; Amisano, G.; Fornasero, S.; Cravotto, G.; Gallicchio, M. Antiproliferative, Proapoptotic, Antioxidant and Antimicrobial Effects of Sinapis nigra L. and Sinapis alba L. Extracts. Molecules 2018, 23, 3004. https://doi.org/10.3390/molecules23113004
Boscaro V, Boffa L, Binello A, Amisano G, Fornasero S, Cravotto G, Gallicchio M. Antiproliferative, Proapoptotic, Antioxidant and Antimicrobial Effects of Sinapis nigra L. and Sinapis alba L. Extracts. Molecules. 2018; 23(11):3004. https://doi.org/10.3390/molecules23113004
Chicago/Turabian StyleBoscaro, Valentina, Luisa Boffa, Arianna Binello, Gabriella Amisano, Stefania Fornasero, Giancarlo Cravotto, and Margherita Gallicchio. 2018. "Antiproliferative, Proapoptotic, Antioxidant and Antimicrobial Effects of Sinapis nigra L. and Sinapis alba L. Extracts" Molecules 23, no. 11: 3004. https://doi.org/10.3390/molecules23113004
APA StyleBoscaro, V., Boffa, L., Binello, A., Amisano, G., Fornasero, S., Cravotto, G., & Gallicchio, M. (2018). Antiproliferative, Proapoptotic, Antioxidant and Antimicrobial Effects of Sinapis nigra L. and Sinapis alba L. Extracts. Molecules, 23(11), 3004. https://doi.org/10.3390/molecules23113004