Cloning, Characterization and Anion Inhibition Studies of a β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba histolytica
Abstract
:1. Introduction
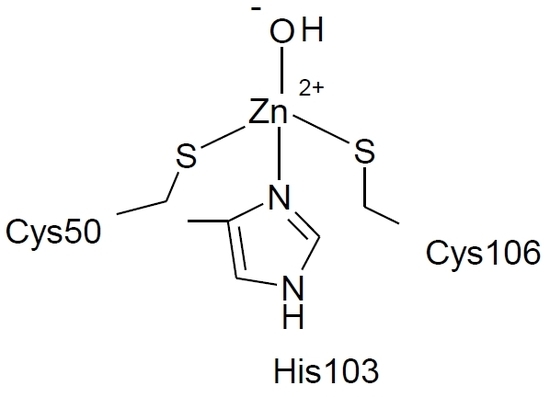
2. Results and Discussion
3. Materials and Methods
3.1. Vector Construction
3.2. Production of the Protein
3.3. CA Activity and Inhibition Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzyme Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Di Fonzo, P.; Carginale, V.; Donald, W.A.; Supuran, C.T.; Capasso, C. Comparison of the sulfonamide inhibition profiles of the β- and γ-carbonic anhydrases from the pathogenic bacterium Burkholderia pseudomallei. Molecules 2017, 22, 421. [Google Scholar] [CrossRef] [PubMed]
- Berrino, E.; Bua, S.; Mori, M.; Botta, M.; Murthy, V.S.; Vijayakumar, V.; Tamboli, Y.; Bartolucci, G.; Mugelli, A.; Cerbai, E.; et al. Novel sulfamide-containing compounds as selective carbonic anhydrase i inhibitors. Molecules 2017, 22, 1049. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.E.; Ashour, D.S.; Abou Rayia, D.M.; Bedeer, A.E. Carbonic anhydrase enzyme as a potential therapeutic target for experimental trichinellosis. Parasitol. Res. 2016, 115, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Langella, E.; Viparelli, F.; Vullo, D.; Ascione, G.; Dathan, N.A.; Morel, F.M.; Supuran, C.T.; De Simone, G.; Monti, S.M. Structural and inhibition insights into carbonic anhydrase CDCA1 from the marine diatom Thalassiosira weissflogii. Biochimie 2012, 94, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Cau, Y.; Mori, M.; Supuran, C.T.; Botta, M. Mycobacterial carbonic anhydrase inhibition with phenolic acids and esters: Kinetic and computational investigations. Org. Biomol. Chem. 2016, 14, 8322–8330. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Rowlett, R.S. Structure and catalytic mechanism of the β-carbonic anhydrases. Biochim. Biophys. Acta Proteins Proteomics 2010, 1804, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari Emameh, R.; Barker, H.; Hytönen, V.P.; Tolvanen, M.E.E.; Parkkila, S. Beta carbonic anhydrases: Novel targets for pesticides and anti-parasitic agents in agriculture and livestock husbandry. Parasites Vect. 2014, 7, 403. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Onishi, S.; Takeuchi, H.; Supuran, C.T. The α and β classes carbonic anhydrases from helicobacter pylori as novel drug targets. Curr. Pharm. Des. 2008, 14, 622–630. [Google Scholar] [PubMed]
- Syrjänen, L.; Parkkila, S.; Scozzafava, A.; Supuran, C.T. Sulfonamide inhibition studies of the β carbonic anhydrase from Drosophila melanogaster. Bioorg. Med. Chem. Lett. 2014, 24, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. An overview of the bacterial carbonic anhydrases. Metabolites 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J. Enzym. Inhib. Med. Chem. 2016, 31, 1254–1260. [Google Scholar] [CrossRef]
- Supuran, C.T.; Capasso, C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin. Ther. Pat. 2018, 28, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Capasso, C.; Supuran, C.T.; Carginale, V. Recombinant thermoactive phosphoenolpyruvate carboxylase (PEPC) from Thermosynechococcus elongatus and its coupling with mesophilic/thermophilic bacterial carbonic anhydrases (CAs) for the conversion of CO2 to oxaloacetate. Bioorg. Med. Chem. 2016, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Bootorabi, F.; Jänis, J.; Smith, E.; Waheed, A.; Kukkurainen, S.; Hytönen, V.; Valjakka, J.; Supuran, C.T.; Vullo, D.; Sly, W.S.; et al. Analysis of a shortened form of human carbonic anhydrase VII expressed in vitro compared to the full-length enzyme. Biochimie 2010, 92, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Vermelho, A.B.; Capaci, G.R.; Rodrigues, I.A.; Cardoso, V.S.; Mazotto, A.M.; Supuran, C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg. Med. Chem. 2017, 25, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Cardoso, V.; Vermelho, A.B.; Ricci Junior, E.; Almeida Rodrigues, I.; Mazotto, A.M.; Supuran, C.T. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J. Enzym. Inhib. Med. Chem. 2018, 33, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Hashmey, N.; Genta, N.; White, N., Jr. Parasites and Diarrhea. I: Protozoans and Diarrhea. J. Travel Med. 1997, 4, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, A.; Erler, H.; Beitz, E. Targeting Channels and Transporters in Protozoan Parasite Infections. Front. Chem. 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Quach, J.; St-Pierre, J.; Chadee, K. The future for vaccine development against Entamoeba histolytica. Hum. Vaccin. Immunother. 2014, 10, 1514–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftus, B.; Anderson, I.; Davies, R.; Alsmark, U.C.M.; Samuelson, J.; Amedeo, P.; Roncaglia, P.; Berriman, M.; Hirt, R.P.; Mann, B.J.; et al. The genome of the protist parasite Entamoeba histolytica. Nature 2005, 433, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Hoffner, R.J.; Kilaghbian, T.; Esekogwu, V.I.; Henderson, S.O. Common presentations of amebic liver abscess. Ann. Emerg. Med. 1999, 34, 351–355. [Google Scholar] [CrossRef]
- Covarrubias, A.S.; Bergfors, T.; Jones, T.A.; Högbom, M. Structural mechanics of the pH-dependent activity of beta-carbonic anhydrase from Mycobacterium tuberculosis. J. Biol. Chem. 2006, 281, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Cloning, characterization, and inhibition studies of a new beta-carbonic anhydrase from Mycobacterium tuberculosis. J. Med. Chem. 2009, 52, 3116–3120. [Google Scholar] [CrossRef] [PubMed]
- Ferraroni, M.; Del Prete, S.; Vullo, D.; Capasso, C.; Supuran, C.T. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- McGurn, L.D.; Moazami-Goudarzi, M.; White, S.A.; Suwal, T.; Brar, B.; Tang, J.Q.; Espie, G.S.; Kimber, M.S. The structure, kinetics and interactions of the β-carboxysomal β-carbonic anhydrase, CcaA. Biochem. J. 2016, 473, 4559–4572. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- De Simone, G.; Supuran, C.T. (In)organic anions as carbonic anhydrase inhibitors. J. Inorg. Biochem. 2012, 111, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Abbate, F.; Supuran, C.T.; Scozzafava, A.; Orioli, P.; Stubbs, M.T.; Klebe, G. Nonaromatic sulfonamide group as an ideal anchor for potent human carbonic anhydrase inhibitors: Role of hydrogen-bonding networks in ligand binding and drug design. J. Med. Chem. 2004, 47, 550–557. [Google Scholar] [CrossRef]
- Murray, A.B.; Aggarwal, M.; Pinard, M.; Vullo, D.; Patrauchan, M.; Supuran, C.T.; McKenna, R. Structural Mapping of Anion Inhibitors to β-Carbonic Anhydrase psCA3 from Pseudomonas aeruginosa. ChemMedChem 2018, 13, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Määttä, J.A.E.; Eisenberg-Domovich, Y.; Nordlund, H.R.; Hayouka, R.; Kulomaa, M.S.; Livnah, O. Chimeric avidin shows stability against harsh chemical conditions—Biochemical analysis and 3D structure. Biotechnol. Bioengin. 2011, 108, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Borras, J.; Scozzafava, A.; Menabuoni, L.; Mincione, F.; Briganti, F.; Mincione, G.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: Is the tail more important than the ring? Bioorg. Med. Chem. 1999, 7, 2397–2406. [Google Scholar] [CrossRef]
- Supuran, C.T.; Clare, B.W. Carbonic anhydrase inhibitors—Part 57: Quantum chemical QSAR of a group of 1,3,4-thiadiazole-and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur. J. Med. Chem. 1999, 34, 41–50. [Google Scholar] [CrossRef]
- Zimmerman, S.A.; Ferry, J.G.; Supuran, C.T. Inhibition of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases. Curr. Top. Med. Chem. 2007, 7, 901–908. [Google Scholar] [CrossRef] [PubMed]
Sample availability: Samples of the compounds described in the paper are available from the authors. |


| Enzyme | Activity Level | Class | kcat (s−1) | Km (mM) | kcat/Km (M−1 × s−1) | KI (Acetazolamide) (nM) |
|---|---|---|---|---|---|---|
| hCA I | moderate | α | 2.0 × 105 | 4.0 | 5.0 × 107 | 250 |
| hCA II | very high | α | 1.4 × 106 | 9.3 | 1.5 × 108 | 2 |
| EhiCA | high | β | (6.7 ± 0.2) × 105 | 7.5 ± 0.08 | (8.9 ± 0.1) × 107 | 509 |
| Inhibitor § | KI [mM] # | ||
|---|---|---|---|
| hCA II | hCA I | EhiCA | |
| F− | >300 | >300 | >100 |
| Cl− | 200 | 6.0 | >100 |
| Br− | 63 | 4.1 | 36.8 |
| I− | 26 | 0.3 | 7.4 |
| CNO− | 0.03 | 0.0007 | 0.77 |
| SCN− | 1.6 | 0.2 | 7.9 |
| CN− | 0.02 | 0.0005 | >100 |
| N3− | 1.51 | 0.0012 | >100 |
| HCO3− | 85 | 12 | 0.28 |
| CO32− | 73 | 15 | 2.4 |
| NO3− | 35 | 7.0 | 3.6 |
| NO2− | 63 | 8.4 | 1.7 |
| HS− | 0.04 | 0.0006 | 6.9 |
| HSO3− | 89 | 18 | 11.5 |
| SO42− | >200 | 63 | 21.6 |
| SnO32− | 0.83 | 0.57 | 0.51 |
| SeO42− | 112 | 118 | 6.0 |
| TeO42− | 0.92 | 0.66 | 0.61 |
| P2O74− | 48.50 | 25.8 | >100 |
| V2O74− | 0.57 | 0.54 | >100 |
| B4O72− | 0.95 | 0.64 | 0.29 |
| ReO4− | 0.75 | 0.11 | 7.1 |
| RuO4− | 0.69 | 0.10 | 7.0 |
| S2O82− | 0.084 | 0.11 | 8.4 |
| SeCN− | 0.086 | 0.085 | 0.87 |
| CS32− | 0.0088 | 0.0087 | 6.0 |
| Et2NCS2− | 3.1 | 0.00079 | 0.51 |
| ClO4− | >200 | >200 | >100 |
| BF4− | >200 | >200 | >100 |
| FSO3− | 0.46 | 0.79 | 0.086 |
| PF6− | >200 | >200 | >100 |
| CF3SO3− | >200 | >200 | >100 |
| NH(SO3)22− | 0.76 | 0.31 | 2.2 |
| H2NSO2NH2 | 1.13 | 0.31 | 0.028 |
| H2NSO3H | 0.39 | 0.021 | >100 |
| Ph-B(OH)2 | 23.1 | 38.6 | 0.047 |
| Ph-AsO3H2 | 49.2 | 31.7 | 0.038 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haapanen, S.; Bua, S.; Kuuslahti, M.; Parkkila, S.; Supuran, C.T. Cloning, Characterization and Anion Inhibition Studies of a β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba histolytica. Molecules 2018, 23, 3112. https://doi.org/10.3390/molecules23123112
Haapanen S, Bua S, Kuuslahti M, Parkkila S, Supuran CT. Cloning, Characterization and Anion Inhibition Studies of a β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba histolytica. Molecules. 2018; 23(12):3112. https://doi.org/10.3390/molecules23123112
Chicago/Turabian StyleHaapanen, Susanna, Silvia Bua, Marianne Kuuslahti, Seppo Parkkila, and Claudiu T. Supuran. 2018. "Cloning, Characterization and Anion Inhibition Studies of a β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba histolytica" Molecules 23, no. 12: 3112. https://doi.org/10.3390/molecules23123112
APA StyleHaapanen, S., Bua, S., Kuuslahti, M., Parkkila, S., & Supuran, C. T. (2018). Cloning, Characterization and Anion Inhibition Studies of a β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba histolytica. Molecules, 23(12), 3112. https://doi.org/10.3390/molecules23123112