Why Natural or Electron Irradiated Sheep Wool Show Anomalous Sorption of Higher Concentrations of Copper(II)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sorption of Cu(II)
2.2. FTIR Analysis of Wool After Contact with Cu(II)
2.3. EPR Spectrometry
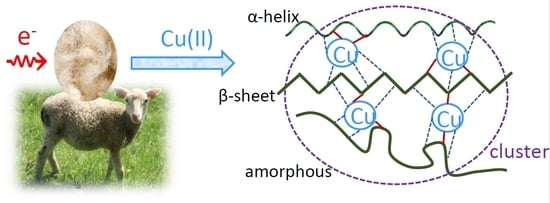
2.4. Assumed Reasons for the Reduced Sorption
3. Materials and Methods
3.1. Materials
3.2. Sheep Wool Scouring and Irradiation
3.3. Batch Sorption Experiments
3.4. Determination of Residual Cu(II) Concentration
3.5. FTIR Spectral Measurements
3.6. EPR Spectral Measurements
3.7. Measurement of pH
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flores-Garnica, J.G.; Morales-Barrera, L.; Pineda-Camacho, G.; Cristiani-Urbina, E. Biosorption of Ni(II) from aqueous solutions by Litchi chinensis seeds. Bioresour. Technol. 2013, 136, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Rosales, E.; Ferreira, L.; Sanromán, M.Á.; Tavares, T.; Pazos, M. Enhanced selective metal adsorption on optimised agroforestry waste mixtures. Bioresour. Technol. 2015, 182, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Pujol, D.; Fiol, N.; Olivella, M.À.; de la Torre, F.; Poch, J.; Villaescusa, I. New Insights into the Role of Chemical Components on Metal Ions Sorption by Grape Stalks Waste. Water Air Soil Pollut. 2015, 226, 1–9. [Google Scholar] [CrossRef]
- Aravind, J.; Lenin, C.; Nancyflavia, C.; Rashika, P.; Saravanan, S. Response surface methodology optimization of nickel (II) removal using pigeon pea pod biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 105–114. [Google Scholar] [CrossRef]
- Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Olumide, A.A.; Bonilla-Petriciolet, A. A survey of multi-component sorption models for the competitive removal of heavy metal ions using bush mango and flamboyant biomasses. J. Mol. Liq. 2016, 224, 1041–1054. [Google Scholar] [CrossRef]
- Radetić, M.; Jocić, D.; Jovančić, P.; Rajaković, L.; Thomas, H.; Petrović, Z.L. Recycled-Wool-Based Nonwoven Material as a Sorbent for Lead Cations. J. Appl. Polym. Sci. 2003, 90, 379–386. [Google Scholar] [CrossRef]
- Radetić, M.; Radojević, D.; Ilić, V.; Jocić, D.; Povrenović, D.; Puač, N.; Petrović, Z.Lj.; Jovančić, P. The Study of Control Parameters for Some Divalent Metal Cations Sorption by Recycled Wool-based Nonwoven Material. Trends Appl. Sci. Res. 2006, 1, 546–574. [Google Scholar]
- Manassra, A.; Khamis, M.; Ihmied, T.; El Dakiky, M. Removal of chromium by continuous flow using wool packed columns. EJEAFChe (Electron. J. Environ. Food Chem.) 2010, 9, 651–663. [Google Scholar]
- Hanzlíková, Z.; Braniša, J.; Hybler, P.; Šprinclová, I.; Jomová, K.; Porubská, M. Sorption properties of sheep wool irradiated by accelerated electron beam. Chem. Pap. 2016, 70, 1299–1308. [Google Scholar] [CrossRef]
- Sheffield, A.; Doyle, M.J. Uptake of Copper(II) by Wool. Text. Res. J. 2005, 75, 203–207. [Google Scholar] [CrossRef]
- Naik, R.; Wen, G.; Dharmaprakash, M.S.; Hureau, S.; Uedono, A.; Liu, W.X.; Cookson, P.G.; Smith, S.V. Metal ion binding properties of novel wool powders. J. Appl. Polym. Sci. 2010, 115, 1642–1650. [Google Scholar] [CrossRef]
- Wen, G.; Naik, R.; Cookson, P.G.; Smith, S.V.; Liu, X.; Wang, X.G. Wool Powders Used as Sorbents to Remove Co2+ Ions from Aqueous Solution. Powder Technol. 2010, 197, 235–240. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Braniša, J.; Jomová, K.; Fülöp, M.; Hybler, P.; Porubská, M. Electron beam irradiated sheep wool—Prospective sorbent for heavy metals in wastewater. Sep. Purif. Technol. 2018, 193, 345–350. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Ueber die Adsorption in Loesungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar]
- Porubská, M.; Hanzlíková, Z.; Braniša, J.; Kleinová, A.; Hybler, P.; Fülöp, M.; Ondruška, J.; Jomová, K. The effect of electron beam on sheep wool. Polym. Degrad. Stabil. 2015, 111, 151–158. [Google Scholar] [CrossRef]
- Milata, V.; Segľa, P.; Brezová, V.; Gatiaľ, A.; Kováčik, V.; Miglierini, M.; Stankovský, Š.; Šíma, J. Aplikovaná molekulová spektroskopia (Applied Molecular Spectroscopy, in Slovak; Publisher STU: Bratislava, Slovak, 2008; pp. 543–558. [Google Scholar]
- Thermo Scientific, Infrared Correlation Chart. Available online: http://ftirsearch.com (accessed on 30 November 2018).
- Liu, H.; Yu, W. Study of the structure transformation of wool fibers with Raman spectroscopy. J. Appl. Polym. Sci. 2007, 103, 1–7. [Google Scholar] [CrossRef]
- Varesano, A.; Vineis, C.; Tonetti, C.; Sánches Ramírez, D.O.; Mazzuchetti, G. Chemical and physical modification of electron keratin nanofibers induced by heating treatments. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Cai, S.W.; Singh, B.H. A Distinct Utility of the Amide III Infrared Band for Secondary Structure Estimation of Aqueous Protein Solutions Using Partial Least Squares Methods. Biochemistry 2004, 43, 2541–2549. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Zimmerman, B.; Chow, J.; Abbott, A.G.; Ellison, M.S.; Kennedy, M.S.; Dean, D. Variation of Surface Charge along the Surface of Wool Fibers Assessed by High-Resolution Force Spectroscopy. J. Eng. Fiber. Fabr. 2011, 6, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kellö, V.; Tkáč, A. Fyzikálna chémia (Physical Chemistry), 2nd ed.; Alfa SNTL: Bratislava, Slovak, 1969. [Google Scholar]
- Baryshnikova, A.T.; Minaev, B.F.; Baryshnikov, G.V.; Sun, W.-H. Quantum-chemical study of the structure and magnetic properties of mono- and binuclear Cu(II) complexes with 1,3-bis(3-(pyrimidin-2-yl)-1H,-1,2,4-triazol-5-yl)propane. Russian J. Inorg. Chem. 2016, 61, 588–593. [Google Scholar] [CrossRef]
- Shulgin, V.F.; Konnik, O.V.; Gusev, A.N.; Boca, R.; Dlhan, L.; Rusanov, E.B.; Alexandrov, G.G.; Eremenko, I.L.; Linert, W. Spacer-armed copper(II) complexes with benzenecarboxylic acids and trifluoroacetylacetone aroylhydrazones. Dalton Trans. 2013, 42, 16878–16886. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikov, G.V.; Minaev, B.F.; Baryshnikova, A.T.; Agren, H. A computational study of structural and magnetic properties of bi- and trinuclear Cu(II) complexes with extremely long Cu---Cu distances. Chem. Phys. 2017, 491, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Baryshnikov, G.V.; Minaev, B.F.; Baryshnikova, A.T.; Agren, H. Anion-induced exchange interactions in binuclear complexes of Cu(II) with flexible hexadentate bispicolylamidrazone ligands. Chem. Phys. Lett. 2016, 661, 48–52. [Google Scholar] [CrossRef]
- Baryshnikova, A.T.; Minaev, B.F.; Baryshnikov, G.V.; Agren, H. Computational study of the structure and magnetic properties of the weakly coupled tetranuclear square-planar complex of Cu(II) with a tetraporphyrin sheet. Inorg. Chim. Acta 2019, 485, 73–79. [Google Scholar] [CrossRef]
- Chesnut, D.B.; Quin, L.D. Nature of Bonding in the Sulfuryl Group. J. Comput. Chem. 2004, 25, 734–738. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Lawson, M.K.; Hybler, P.; Fülöp, M.; Porubská, M. Time-Dependent Variations in Structure of Sheep Wool Irradiated by Electron Beam. Adv. Mater. Sci. Eng. 2017, 2017. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porubská, M.; Kleinová, A.; Hybler, P.; Braniša, J. Why Natural or Electron Irradiated Sheep Wool Show Anomalous Sorption of Higher Concentrations of Copper(II). Molecules 2018, 23, 3180. https://doi.org/10.3390/molecules23123180
Porubská M, Kleinová A, Hybler P, Braniša J. Why Natural or Electron Irradiated Sheep Wool Show Anomalous Sorption of Higher Concentrations of Copper(II). Molecules. 2018; 23(12):3180. https://doi.org/10.3390/molecules23123180
Chicago/Turabian StylePorubská, Mária, Angela Kleinová, Peter Hybler, and Jana Braniša. 2018. "Why Natural or Electron Irradiated Sheep Wool Show Anomalous Sorption of Higher Concentrations of Copper(II)" Molecules 23, no. 12: 3180. https://doi.org/10.3390/molecules23123180
APA StylePorubská, M., Kleinová, A., Hybler, P., & Braniša, J. (2018). Why Natural or Electron Irradiated Sheep Wool Show Anomalous Sorption of Higher Concentrations of Copper(II). Molecules, 23(12), 3180. https://doi.org/10.3390/molecules23123180