Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications
Abstract
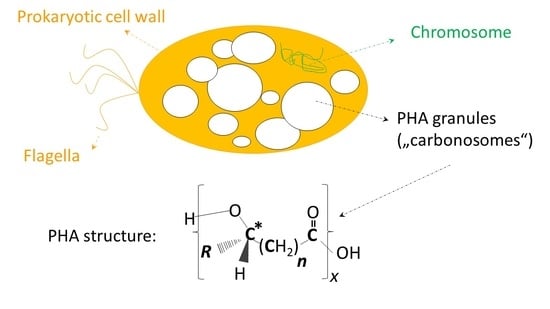
:1. Introduction
2. Biocompatibility Aspects and Purity Requirements for PHA to be used In Vivo
3. Drug Encapsulation in PHA Carriers for Controlled Liberation of Bioactive Compounds
3.1. General
3.2. PHA-Based Micro- and Nanocarriers
4. PHA-Based Implants, Sutures and Scaffolds for Tissue Engineering and Tissue Repair
4.1. PHA-Based Implants
4.2. PHA in Tissue Engineering
4.3. PHA-Sutures for Muscle and Skin Regeneration
4.4. PHA in Blood Vessel Regeneration
4.5. PHA in Cartilage Repair
4.6. PHA in Nerve Repair
5. Conclusions
Conflicts of Interest
References
- Jendrossek, D. Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J. Bacteriol. 2009, 191, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2017. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, E.; Sedlacek, P.; Mravec, F.; Mullerova, L.; Samek, O.; Koller, M.; Hesko, O.; Kucera, D.; Marova, I.; Obruca, S. Light scattering on PHA granules protects bacterial cells against the harmful effects of UV radiation. Appl. Microbiol. Biotechnol. 2018, 102, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Maršálek, L.; Miranda de Sousa Dias, M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.; Reis, M.A. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Adamus, G.; Kurcok, P.; Radecka, I.; Kowalczuk, M. Bioactive oligomers from natural polyhydroxyalkanoates and their synthetic analogues. Polimery 2017, 62, 317–322. [Google Scholar] [CrossRef]
- Utsunomia, C.; Saito, T.; Matsumoto, K.I.; Hori, C.; Isono, T.; Satoh, T.; Taguchi, S. Synthesis of lactate (LA)-based poly(ester-urethane) using hydroxyl-terminated LA-based oligomers from a microbial secretion system. J. Polym. Res. 2017, 24, 167. [Google Scholar] [CrossRef]
- Shahzad, K.; Narodoslawsky, M.; Sagir, M.; Ali, N.; Ali, S.; Rashid, M.I.; Ismail, I.M.I.; Koller, M. Techno-economic feasibility of waste biorefinery: Using slaughtering waste streams as starting material for biopolyester production. Waste Manag. 2017, 67, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Pittmann, T.; Steinmetz, H. Polyhydroxyalkanoate Production on waste water treatment plants: Process scheme, operating conditions and potential analysis for German and European municipal waste water treatment plants. Bioengineering 2017, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2014, 39, 397–442. [Google Scholar] [CrossRef]
- Lefebvre, G.; Rocher, M.; Braunegg, G. Effects of low dissolved-oxygen concentrations on poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Alcaligenes eutrophus. Appl. Environ. Microbiol. 1997, 63, 827–833. [Google Scholar] [PubMed]
- Koller, M.; Bona, R.; Chiellini, E.; Fernandes, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Fasl, H.; Stelzer, F.; Braunegg, G. Study on the effect of levulinic acid on whey-based biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Hydrogenophaga pseudoflava. Appl. Food Biotechnol. 2017, 4, 65–78. [Google Scholar] [CrossRef]
- Koller, M.; Miranda de Sousa Dias, M.; Rodríguez-Contreras, A.; Kunaver, M.; Žagar, E.; Kržan, A.; Braunegg, G. Liquefied wood as inexpensive precursor-feedstock for bio-mediated incorporation of (R)-3-hydroxyvalerate into polyhydroxyalkanoates. Materials 2015, 8, 6543–6557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, M.; Salerno, A.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Illieva, V.; Chiellini, E.; Braunegg, G. Novel precursors for production of 3-hydroxyvalerate-containing poly[(R)-hydroxyalkanoate]s. Biocatal. Biotransform. 2014, 32, 161–167. [Google Scholar] [CrossRef]
- Miranda de Sousa Dias, M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-Batch synthesis of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from sucrose and 4-hydroxybutyrate precursors by Burkholderia sacchari strain DSM 17165. Bioengineering 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.S.; de Almeida, M.C.M.; da Fonseca, M.M.R.; Cesário, M.T. Feeding strategies for tuning poly(3-hydroxybutyrate-co-4-hydroxybutyrate) monomeric composition and productivity using Burkholderia sacchari. Int. J. Biol. Macromol. 2017, 105, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of high quality polyhydroxyalkanoate co-and terpolyesters for potential medical application by the archaeon Haloferax mediterranei. Macromol. Symp. 2007, 253, 33–39. [Google Scholar] [CrossRef]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Brigham, C.J.; Sinskey, A.J. Applications of polyhydroxyalkanoates in the medical industry. Int. J. Biotechnol. Wellness Ind. 2012, 1, 52–60. [Google Scholar] [CrossRef]
- Junyu, Z.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, in press. [Google Scholar] [CrossRef]
- Luef, K.P.; Stelzer, F.; Wiesbrock, F. Poly(hydroxyalkanoate)s in medical applications. Chem. Biochem. Eng. Q. 2015, 29, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.A. Applications of PHB-a microbially produced biodegradable thermoplastic. Phys. Technol. 1985, 16, 32–36. [Google Scholar] [CrossRef]
- Tan, D.; Yin, J.; Chen, G.Q. Production of Polyhydroxyalkanoates. In Current Developments in Biotechnology and Bioengineering, Production, Isolation and Purification of Industrial Products; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 655–692. ISBN 978-0-444-63662-1. [Google Scholar]
- Jost, V.; Kopitzky, R. Blending of polyhydroxybutyrate-co-valerate with polylactic acid for packaging applications–reflections on miscibility and effects on the mechanical and barrier properties. Chem. Biochem. Eng. Q. 2015, 29, 221–246. [Google Scholar] [CrossRef]
- Pérez Amaro, L.; Chen, H.; Barghini, A.; Corti, A.; Chiellini, E. High performance compostable biocomposites based on bacterial polyesters suitable for injection molding and blow extrusion. Chem. Biochem. Eng. Q. 2015, 29, 261–274. [Google Scholar] [CrossRef]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, M.; Roes, L.; Patel, M.K.; Chiellini, E. Comparative life cycle studies on poly(3-hydroxybutyrate)-based composites as potential replacement for conventional petrochemical plastics. Biomacromolecules 2007, 8, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.P. Applications of PHAs in medicine and pharmacy. In Biopolymers Polyesters III-Applications and Commercial Products; Doi, Y., Steinbüchel, A., Eds.; Wiley-VCH: Chichester, UK, 2002; pp. 91–127. [Google Scholar]
- Hufenus, R.; Reifler, F.A.; Maniura-Weber, K.; Spierings, A.; Zinn, M. Biodegradable bicomponent fibers from renewable sources: Melt-spinning of poly(lactic acid) and poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)]. Macromol. Mater. Eng. 2012, 297, 75–84. [Google Scholar] [CrossRef]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Mohapatra, S.; Maity, S.; Dash, H.R.; Das, S.; Pattnaik, S.; Rath, C.C.; Samantaray, D. Bacillus and biopolymer: Prospects and challenges. Biochem. Biophys. Rep. 2017, 12, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Teeka, J.; Imai, T.; Reungsang, A.; Cheng, X.; Yuliani, E.; Thiantanankul, J.; Poomipuk, N.; Yamaguchi, J.; Jeenanong, A.; Higuchi, T.; et al. Characterization of polyhydroxyalkanoates (PHAs) biosynthesis by isolated Novosphingobium sp. THA_AIK7 using crude glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Sevastianov, V.I.; Perova, N.V.; Shishatskaya, E.I.; Kalacheva, G.S.; Volova, T.G. Production of purified polyhydroxyalkanoates (PHAs) for applications in contact with blood. J. Biomater. Sci. Polym. Ed. 2003, 21, 1029–1042. [Google Scholar] [CrossRef]
- Wampfler, B.; Ramsauer, T.; Rezzonico, S.; Hischier, R.; Kohling, R.; Thony-Meyer, L.; Zinn, M. Isolation and purification of medium chain length poly(3-hydroxyalkanoates) (mcl-PHA) for medical applications using nonchlorinated solvents. Biomacromolecules 2010, 11, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzochetti, L.; Torri, C.; Tagliavini, E. Dimethyl carbonate and switchable anionic surfactants: Two effective tools for the extraction of polyhydroxyalkanoates from microbial biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Riedel, S.L.; Brigham, C.J.; Budde, C.F.; Bader, J.; Rha, C.; Stahl, U.; Sinskey, A.J. Recovery of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from Ralstonia eutropha cultures with non-halogenated solvents. Biotechnol. Bioeng. 2013, 110, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.; Bharmoria, P.; Gehlot, P.S.; Agrawal, V.; Kumar, A.; Mishra, S. 1-Ethyl-3-methylimidazolium diethylphosphate based extraction of bioplastic “Polyhydroxyalkanoates” from bacteria: Green and Sustainable Approach. ACS Sustain. Chem. Eng. 2018, 6, 766–773. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.W.; Wei, Y.H.; Wu, H.S.; Wang, S.S. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Madkour, M.H.; Heinrich, D.; Alghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. PHA recovery from biomass. Biomacromolecules 2013, 14, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Bona, R.; Chiellini, E.; Braunegg, G. Extraction of short-chain-length poly-[(R)-hydroxyalkanoates] (scl-PHA) by the “anti-solvent” acetone under elevated temperature and pressure. Biotechnol. Lett. 2013, 35, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.H.; Shen, C.Y.; You, M.L.; Xiao, J.F.; Chen, G.Q. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials 2010, 31, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Darani, K.; Vasheghani-Farahani, E.; Shojaosadati, S.A.; Yamini, Y. Effect of process variables on supercritical fluid disruption of Ralstonia eutropha cells for poly(R-hydroxybutyrate) recovery. Biotechnol. Prog. 2004, 20, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Daly, S.R.; Fathi, A.; Bahramian, B.; Manavitehrani, I.; McClure, D.D.; Valtchev, P.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. A green process for the purification of biodegradable poly(β-hydroxybutyrate). J. Supercrit. Fluids 2018, 135, 84–90. [Google Scholar] [CrossRef]
- Korsatko, W.; Wabnegg, B.; Tillian, H.M.; Egger, G. Poly-d(-)-3-Hydroxybuttersäure—Ein biologisch abbaubarer Arzneistoffträger zur Liberationsverzögerung. 3. Mitt.: Gewebsverträglichkeitsstudien parental applizierbarer Poly-d(−)-3-Hydroxybuttersäure-Tabletten in Gewebekultur und in vivo. Pharm. Ind. 1984, 46, 952–954. (In German) [Google Scholar]
- Shishatskaya, E.I.; Volova, T.; Puzyr, A.P.; Mogilnaya, O.A.; Efremov, S.N. Tissue response to the implantation of biodegradable polyhydroxyalkanoate sutures. J. Mater. Sci. Mater. Med. 2004, 15, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Wu, Q.; Chen, G.Q. Gelatin blending improves the performance of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) films for biomedical application. Biomacromolecules 2005, 6, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R.; Nigmatullin, R.; Taylor, C.; Haycock, J.W.; Claeyssens, F.; Knowles, J.C.; Roy, I. Nerve tissue engineering using blends of poly(3-hydroxyalkanoates) for peripheral nerve regeneration. Eng. Life Sci. 2015, 15, 612–621. [Google Scholar] [CrossRef]
- Nobes, G.A.R.; Maysinger, D.; Marchessault, R.H. Polyhydroxyalkanoates: Materials for delivery systems. Drug Deliv. 1998, 5, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Rezaie Shirmard, L.; Bahari Javan, N.; Khoshayand, M.R.; Kebriaee-Zadeh, A.; Dinarvand, R.; Dorkoosh, F.A. Nanoparticulate fingolimod delivery system based on biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Design, optimization, characterization and in vitro evaluation. Pharm. Dev. Technol. 2017, 22, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Kassab, A.C.; Piskin, E.; Bilgic, S.; Denkbas, E.B.; Xu, K. Embolization with polyhydroxybutyrate (PHB) microspheres: In vivo studies. J. Bioact. Compat. Polym. 1999, 14, 291–303. [Google Scholar] [CrossRef]
- Kassab, A.C.; Xu, K.; Denkbas, E.B.; Dou, Y.; Zhao, S.; Piskin, E. Rifampicin carrying polyhydroxybutyrate microspheres as a potential chemoembolization agent. J. Biomater. Sci. Polym. Ed. 1997, 8, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Sendil, D.; Gürsel, I.; Wise, D.L.; Hasırcı, V. Antibiotic release from biodegradable PHBV microparticles. J. Control. Release 1999, 59, 207–217. [Google Scholar] [CrossRef]
- Xiong, Y.C.; Yao, Y.C.; Zhan, X.Y.; Chen, G.Q. Application of polyhydroxyalkanoates nanoparticles as intracellular sustained drug-release vectors. J. Biomater. Sci. Polym. Ed. 2010, 21, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Naveen, N.; Kumar, R.; Balaji, S.; Uma, T.S.; Natrajan, T.S.; Sehgal, P.K. Synthesis of nonwoven nanofibers by electrospinning—A promising biomaterial for tissue engineering and drug delivery. Adv. Eng. Mater. 2010, 12, B380–B387. [Google Scholar] [CrossRef]
- Gursel, I.; Yagmurlu, F.; Korkusuz, F.; Hasirci, V. In vitro antibiotic release from poly(3-hydroxybutyrate-co-3-hydroxyvalerate) rods. J. Microencapsul. 2002, 19, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Türesin, F.; Gürsel, I.; Hasirci, V. Biodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: In vitro antibiotic release. J. Biomater. Sci. Polym. Ed. 2001, 21, 195–207. [Google Scholar] [CrossRef]
- Scheithauer, E.C.; Li, W.; Ding, Y.; Harhaus, L.; Roether, J.A.; Boccaccini, A.R. Preparation and characterization of electrosprayed daidzein–loaded PHBV microspheres. Mater. Lett. 2015, 158, 66–69. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Kulkarni, A.R.; Aminabhavi, T.M. Blend microspheres of poly(3-hydroxybutyrate) and cellulose acetate phthalate for colon delivery of 5-fluorouracil. Ind. Eng. Chem. Res. 2011, 50, 10414–10423. [Google Scholar] [CrossRef]
- Masood, F.; Chen, P.; Yasin, T.; Fatima, N.; Hasan, F.; Hameed, A. Encapsulation of Ellipticine in poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) based nanoparticles and its in vitro application. Mater. Sci. Eng. C 2013, 33, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Wang, D.; Parhamifar, L.; Hall, A.; Chen, G.Q.; Moghimi, S.M. Poly(3-hydroxybutyrate-co-R-3-hydroxyhexanoate) nanoparticles with polyethylenimine coat as simple, safe, and versatile vehicles for cell targeting: Population characteristics, cell uptake, and intracellular trafficking. Adv. Healthc. Mater. 2014, 3, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Meischel, M.; Eichler, J.; Martinelli, E.; Karr, U.; Weigel, J.; Schmöller, G.; Tschegg, E.K.; Fischerauer, S.; Weinberg, A.M.; Stanzl-Tschegg, S.E. Adhesive strength of bone-implant interfaces and in vivo degradation of PHB composites for load-bearing applications. J. Mech. Behav. Biomed. 2016, 53, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.; Meng, D.; Locke, I.C.; Knowles, J.C.; Mordan, N.; Salih, V.; Boccaccini, A.R.; Roy, I. Novel poly(3-hydroxybutyrate) composite films containing bioactive glass nanoparticles for wound healing applications. Polym. Int. 2016, 65, 661–674. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, M.; Zhang, J.; Zhang, Y.; Liu, Z.; Zhu, Y.; Zhang, C. Three dimensionally printed mesoporous bioactive glass and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) composite scaffolds for bone regeneration. J. Mater. Chem. B 2014, 2, 6106–6118. [Google Scholar] [CrossRef]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.P.; Horowitz, D.M.; Peoples, O.P. PHA applications: Addressing the price performance issue: I. Tissue engineering. Int. J. Biol. Macromol. 1999, 25, 111–121. [Google Scholar] [CrossRef]
- Ellis, G.; Cano, P.; Jadraque, M.; Martín, M.; López, L.; Núñez, T.; de la Peña, E.; Marco, C.; Garrido, L. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: An infrared microspectroscopy study. Anal. Bioanal. Chem. 2011, 399, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Williams, S.F. Medical applications of poly-4-hydroxybutyrate: A strong flexible absorbable biomaterial. Biochem. Eng. J. 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Moore, E.; Soares, J.S.; Rajagopal, K.R. Biodegradable stents: Biomechanical modeling challenges and opportunities. Cardiovasc. Eng. Technol. 2010, 1, 52–65. [Google Scholar] [CrossRef]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.; Locke, I.C.; Keshavarz, T.; Roy, I. Novel poly(3-hydroxyoctanoate)/poly(3-hydroxybutyrate) blends for medical applications. React. Funct. Polym. 2013, 73, 1340–1348. [Google Scholar] [CrossRef]
- Puppi, D.; Pirosa, A.; Lupi, G.; Erba, P.A.; Giachi, G.; Chiellini, F. Design and fabrication of novel polymeric biodegradable stents for small caliber blood vessels by computer-aided wet-spinning. Biomed. Mater. 2017, 12, 035011. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Morelli, A.; Chiellini, F. Additive manufacturing of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) blend scaffolds for tissue engineering. Bioengineering 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, K.; Zhang, X.; Hu, P.; Chen, G.Q. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.Q. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Deng, Y.; Lin, X.-S.; Zheng, Z.; Deng, J.-G.; Chen, J.-C.; Ma, H.; Chen, G.Q. Poly(hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Biomaterials 2003, 24, 4273–4281. [Google Scholar] [CrossRef]
- Lee, I.S.; Kwon, O.H.; Meng, W.; Kang, I.K. Nanofabrication of microbial polyester by electrospinning promotes cell attachment. Macromol. Res. 2004, 12, 374–378. [Google Scholar] [CrossRef]
- Mota, C.; Wang, S.Y.; Puppi, D.; Gazzarri, M.; Migone, C.; Chiellini, F.; Chen, G.Q.; Chiellini, E. Additive manufacturing of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] scaffolds for engineered bone development. J. Tissue Eng. Regen. Med. 2017, 11, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Pirosa, A.; Morelli, A.; Chiellini, F. Design, fabrication and characterization of tailored poly [(R)-3-hydroxybutyrate-co-(R)-3-hydroxyexanoate] scaffolds by computer-aided wet-spinning. Rapid Prototyp. J. 2018, in press. [Google Scholar] [CrossRef]
- Bian, Y.Z.; Wang, Y.; Aibaidoula, G.; Chen, G.Q.; Wu, Q. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials 2009, 30, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Wiberg, M.; Terenghi, G. Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. Br. J. Plast. Surg. 2002, 55, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Mohanna, P.N.; Terenghi, G.; Wiberg, M. Composite PHB–GGF conduit for long nerve gap repair: A long-term evaluation. Scand. J. Plast. Reconstr. 2005, 39, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Atlić, A.; Koller, M.; Scherzer, D.; Kutschera, C.; Grillo-Fernandes, E.; Horvat, P.; Chiellini, E.; Braunegg, G. Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl. Microbiol. Biotechnol. 2011, 91, 295–304. [Google Scholar] [CrossRef] [PubMed]

| Type of PHA | Application | Ref. |
|---|---|---|
| Poly(3-hydroxybutyrate) (PHB) (Homopolyester; scl-PHA) | Tissue compatibility studies of parenteral PHB tablets in mice fibroblast (nota bene: PHB was presumably not of high purity) | [49] |
| Study of physiological and biochemical reactions of rats implanted with PHB sutures | [34] | |
| Preparation of highly pure PHB | [37,47,48] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBHV) (Copolyester; scl-PHA) | Biocompatibility tests of PHBHV/PLA fibers | [33] |
| Blood coagulation, complement reaction, and hemostasis tests | [36] | |
| Study of physiological and biochemical reactions of rats implanted with PHBHV sutures | [34,50] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) (PHB4HBHV) (Terpolyester; scl-PHA) | Preparation of highly pure PHB4HBHV | [45] |
| Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) (Copolyester; scl-mcl-PHA) | Viability of mouse osteoblast cells on PHBHHx films and films of PHBHHx and gelatin | [51] |
| Poly(3-hydroxyoctanoate) (PHO) (Homopolyester; scl-PHA) | Biocompatibility studies with NG108-15 neuronal cells for nerve tissue engineering | [52] |
| Poly(3-hydroxyhexanoate-co-3-hydroxyoctanoate) (PHHxHO) (Copolyester; mcl-PHA) | Preparation of highly pure PHHxHO with low endotoxin levels | [39,46] |
| Poly(3-hydroxy-ω-undecenoate-co-3-hydroxy-ω-nonenoate-co-3-hydroxy-ω-heptenoate) (Copolyester; mcl-PHA) | Preparation of highly pure unsaturated PHA with low endotoxin levels | [39] |
| Type of PHA | Application | Ref. |
|---|---|---|
| Poly(3-hydroxybutyrate) (PHB)(Homopolyester; scl-PHA) | Release of rifampicin immobilized in PHA microspheres | [57] |
| Sustained rhodamine B isothiocyanate release by macrophage endocytosis | [59] | |
| Nanofibrous scaffolds for kanamycin release to prevent infection by Staphylococcus aureus | [60] | |
| In-colon delivery of the anticancer drug 5-fluorouracil from PHB/cellulose acetate phthalate microspheres prepared by solvent casting | [64] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBHV)(Copolyester; scl-PHA) | Release of tetracycline immobilized in PHBHV microspheres and microcapsules | [58] |
| PHBHV rods loaded with sulbactam:cefoperazone and gentamicin for sustained antibiotic release | [61] | |
| PHBHV/PVA nanospheres for in-colon delivery of the anticancer drug 5-fluorouracil | [64] | |
| PHBHV/PVA nanospheres loaded with fingolimod to treat multiple sclerosis | [54] | |
| PHBHV nanospheres coated with PVA for release of antineoplastic drug ellipticine (cancer therapy) | [65] | |
| Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (PHB4HB)(Copolyester; scl-PHA) | Local release of antibiotics Sulperazone® and Duocid® for treatment of chronic osteomyelitis | [62] |
| Microspheres loaded with the phytoestrogen daidzein prepared by electrospraying for osteoporosis hormone therapy | [63] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx)(Copolyester; scl-mcl-PHA) | Sustained rhodamine B isothiocyanate release by macrophage endocytosis | [59] |
| Rhodamine-B-loaded PHBHHx nanoparticles coated with poly(ethylene imine) to study ex vivo and in vivo cell response | [66] | |
| Poly(3-hydroxyoctanoate) (PHO)(Homopolyester; scl-PHA) | Biocompatibility studies with NG108-15 neuronal cells for nerve tissue engineering | [52] |
| Type of PHA | Application | Ref. |
|---|---|---|
| Poly(3-hydroxybutyrate) (PHB) (Homopolyester; scl-PHA) | Bioactive glass nanoparticles embedded in PHB microsphere films for skin regeneration | [68] |
| Guidance conduit channels for long-gap bridging in peripheral nerves in rabbit model | [86,87] | |
| Investigating biomechanical properties, osteoinduction, and in vivo degradability of PHB-ZrO2-Herafill® implants in rat model | [67] | |
| Blends of PHB and PHO for preparation of blood vessel stents | [75] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) (Copolyester; scl-mcl-PHA) | PHBHHx/PCL blends prepared by computer-aided wet-spinning for production of small caliber blood vessel stents | [76] |
| PHBHHx/PHB blends as scaffolds for chondrocytes proliferation | [78,79,80,81] | |
| PHBHHx scaffolds prepared by computer-aided wet-spinning for pre-osteoblast proliferation to osteoblasts | [77] | |
| Conduits for peripheral nerve tissue engineering in rat model experiment | [85] | |
| Scaffolds for differentiation of human bone marrow mesenchymal stem cells | [46] | |
| 3D-scaffolds of composites of PHBHHx and mesoporous bioactive glass for bone regeneration | [69] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) (PHBHVHHx) (Terpolyester; scl-mcl-PHA) | Scaffolds for differentiation of human bone marrow mesenchymal stem cells | [46] |
| Poly(4-hydroxybutyrate) (P4HB) (Homopolyester; scl-PHA) | Highly tensile and strong suture material (TephaFLEX®) | [23] |
| Poly(3-hydroxyoctanoate) (PHO) (Homopolyester; scl-PHA) | Blends of PHB and PHO for preparation of blood vessel stents | [75] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. https://doi.org/10.3390/molecules23020362
Koller M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules. 2018; 23(2):362. https://doi.org/10.3390/molecules23020362
Chicago/Turabian StyleKoller, Martin. 2018. "Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications" Molecules 23, no. 2: 362. https://doi.org/10.3390/molecules23020362
APA StyleKoller, M. (2018). Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules, 23(2), 362. https://doi.org/10.3390/molecules23020362