Half-Sandwich Ru(II) and Os(II) Bathophenanthroline Complexes Containing a Releasable Dichloroacetato Ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. 1H NMR Spectroscopy and ESI+ Mass Spectrometry Studies of Hydrolytic Stability
2.3. Mass Spectrometry Studies of Interactions with Sulfur-Containing Biomolecules
2.4. Mass Spectrometry Studies of Interactions with Model Proteins
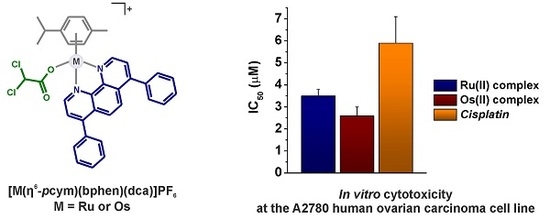
2.5. In Vitro Cytotoxicity
2.6. Cell Cycle Analysis
2.7. Induction of Mitochondrial Membrane Potential Changes and Cytochrome c Release
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Methods
3.4. 1H NMR and ESI+ MS Studies of Hydrolytic Stability
3.5. Studies of Interactions with Biomolecules
3.6. Cell Culture
3.6.1. In Vitro Cytotoxicity
3.6.2. Cellular Accumulation
3.7. Flow Cytometry Studies
3.7.1. Cell Cycle Analysis
3.7.2. Mitochondrial Membrane Potential Assay
3.7.3. Cytochrome c Release
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Song, Y.; Wang, J.; Zhang, C.; Chang, C.; Yan, J.; Qiu, L.; Wua, M.; Guo, Z. A platinum anticancer theranostic agent with magnetic targeting potential derived from maghemite nanoparticles. Chem. Sci. 2013, 4, 2605–2612. [Google Scholar] [CrossRef]
- Boulikas, T. Clinical overview on Lipoplatin: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18, 1197–1218. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Lippard, S.J. Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proc. Natl. Acad. Sci. USA 2009, 106, 22199–22204. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, X.; Mao, W.; Sui, M.; Tang, J.; Shen, Y. Conjugate of Pt(IV)–histone deacetylase inhibitor as a prodrug for cancer chemotherapy. Mol. Pharm. 2012, 9, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, R.; Braude, J.P.; Wexselblatt, E.; Novohradsky, V.; Stuchlikova, O.; Brabec, V.; Gandin, V.; Gibson, D. Pt(IV) derivatives of cisplatin and oxaliplatin with phenylbutyrate axial ligands are potent cytotoxic agents that act by several mechanisms of action. Chem. Sci. 2016, 7, 2381–2391. [Google Scholar] [CrossRef]
- Awuah, S.G.; Zheng, Y.R.; Bruno, P.M.; Hemann, M.T.; Lippard, S.J. A Pt(IV) pro-drug preferentially targets indoleamine-2,3-dioxygenase, providing enhanced ovarian cancer immuno-chemotherapy. J. Am. Chem. Soc. 2015, 137, 14854–14857. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Babak, M.V.; Hartinger, C.G. Development of anticancer agents: Wizardry with osmium. Drug Discov. Today 2014, 19, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Trondl, R.; Heffeter, P.; Kowol, C.R.; Jakupec, M.A.; Berger, W.; Keppler, B.K. NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application. Chem. Sci. 2014, 5, 2925–2932. [Google Scholar] [CrossRef]
- Morris, R.E.; Aird, R.E.; del Socorro Murdoch, P.; Chen, H.; Cummings, J.; Hughes, N.D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D.I.; et al. Inhibition of cancer cell growth by ruthenium(II) arene complexes. J. Med. Chem. 2001, 44, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.F.A.; Habtemariam, A.; Fernández, R.; Walland, V.; Fabbiani, F.P.A.; Parsons, S.; Aird, R.E.; Jodrell, D.I.; Sadler, P.J. Tuning the reactivity of osmium(II) and ruthenium(II) arene complexes under physiological conditions. J. Am. Chem. Soc. 2006, 128, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Romero-Canelón, I.; Mos, M.; Sadler, P.J. Enhancement of selectivity of an organometallic anticancer agent by redox modulation. J. Med. Chem. 2015, 58, 7874–7880. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Barreda, J.J.; Romero-Canelón, I.; Habtemariam, A.; Sadler, P.J. Transfer hydrogenation catalysis in cells as a new approach to anticancer drug design. Nat. Commun. 2015, 6, 6582. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, T.; Twentyman, P.R. A study of ethacrynic acid as a potential modifier of melphalan and cisplatin sensitivity in human lung cancer parental and drug-resistant cell lines. Br. J. Cancer 1992, 65, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Agonigi, G.; Riedel, T.; Gay, M.P.; Biancalana, L.; Oñate, E.; Dyson, P.J.; Pampaloni, G.; Păunescu, E.; Esteruelas, M.A.; Marchetti, F. Arene osmium complexes with ethacrynic acid-modified ligands: Synthesis, characterization, and evaluation of intracellular glutathione S-transferase inhibition and antiproliferative activity. Organometallics 2016, 35, 1046–1056. [Google Scholar] [CrossRef]
- Madhok, B.M.; Yeluri, S.; Perry, S.L.; Hughes, T.A.; Jayne, D.G. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. Br. J. Cancer 2010, 102, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, T.C.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Webster, L.; Mackey, J.R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 2008, 99, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Betanzos-Lara, S.; Novakova, O.; Deeth, R.J.; Pizarro, A.M.; Clarkson, G.J.; Liskova, B.; Brabec, V.; Sadler, P.J.; Habtemariam, A. Bipyrimidine ruthenium(II) arene complexes: Structure, reactivity and cytotoxicity. J. Biol. Inorg. Chem. 2012, 17, 1033–1051. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in cancer cell death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Salemi, G.; Gueli, M.C.; D’Amelio, M.; Saia, V.; Mangiapane, P.; Aridon, P.; Ragonese, P.; Lupo, I. Blood levels of homocysteine, cysteine, glutathione, folic acid, and vitamin B12 in the acute phase of atherothrombotic stroke. Neurol. Sci. 2009, 30, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Koreeda, T.; Kochi, T.; Kakiuchi, F. Ruthenium-catalyzed reductive deamination and tandem alkylation of aniline derivatives. J. Organomet. Chem. 2013, 741–742, 148–152. [Google Scholar] [CrossRef]
- Toohey, J.I.; Cooper, A.J.L. Thiosulfoxide (Sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Messori, L.; Merlino, A. The X-ray structure of the primary adducts formed in the reaction between cisplatin and cytochrome c. Chem. Commun. 2015, 51, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Du, Y.; Cui, M.; Xing, J.; Liu, Z.; Liu, S. Probing the interaction of cisplatin with cytochrome c by electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2012, 84, 6206–6212. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Gabbiani, C.; Michelucci, E.; Pieraccini, G.; Moneti, G.; Dyson, P.J.; Messori, L. Exploring metallodrug-protein interactions by mass spectrometry: Comparisons between platinum coordination complexes and an organometallic ruthenium compound. J. Biol. Inorg. Chem. 2009, 14, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bella, J.; Parkinson, J.A.; Sadler, P.J. Competitive reactions of a ruthenium arene anticancer complex with histidine, cytochrome c and an oligonucleotide. J. Biol. Inorg. Chem. 2005, 10, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, C.; Chaplin, A.B.; Hartinger, C.G.; Bergamo, A.; Cocchietto, M.; Keppler, B.K.; Sava, G.; Dyson, P.J. Tuning the hydrophobicity of ruthenium(II)–arene (RAPTA) drugs to modify uptake, biomolecular interactions and efficacy. Dalton Trans. 2007, 5065–5072. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.S.; Santos, F.C.; Jorge, T.F.; Côrte-Real, L.; Madeira, P.J.A.; Marques, F.; Robalo, M.P.; Matos, A.; Santos, I.; Garcia, M.H. New water-soluble ruthenium(II) cytotoxic complex: Biological activity and cellular distribution. J. Inorg. Biochem. 2014, 130, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Battistin, F.; Scaletti, F.; Balducci, G.; Pillozzi, S.; Arcangeli, A.; Messori, L.; Alessio, E. Water-soluble Ru(II)- and Ru(III)-halide-PTA complexes (PTA = 1,3,5-triaza-7-phosphaadamantane): Chemical and biological properties. J. Inorg. Biochem. 2016, 160, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Mastrobuoni, G.; Ang, W.H.; Gabbiani, C.; Pieraccini, G.; Moneti, G.; Dyson, P.J.; Messori, L. ESI–MS characterisation of protein adducts of anticancer ruthenium(II)-arene PTA (RAPTA) complexes. ChemMedChem 2007, 2, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; Groessl, M.; Meier, S.M.; Kingston, R.L.; Goldstone, D.C.; Hartinger, C.G. The metalation of hen egg white lysozyme impacts protein stability as shown by ion mobility mass spectrometry, differential scanning calorimetry, and X-ray crystallography. Chem. Commun. 2017, 53, 4246–4249. [Google Scholar] [CrossRef] [PubMed]
- Cinellu, M.A.; Maiore, L.; Manassero, M.; Casini, A.; Arca, M.; Fiebig, H.H.; Kelter, G.; Michelucci, E.; Pieraccini, G.; Gabbiani, C.; et al. [Au2(phen2Me)2(μ-O)2](PF6)2, a novel dinuclear gold(III) complex showing excellent antiproliferative properties. ACS Med. Chem. Lett. 2010, 1, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Serratrice, M.; Maiore, L.; Zucca, A.; Stoccoro, S.; Landini, I.; Mini, E.; Massai, L.; Ferraro, G.; Merlino, A.; Messori, L.; et al. Cytotoxic properties of a new organometallic platinum(II) complex and its gold(I) heterobimetallic derivatives. Dalton Trans. 2016, 45, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Groessl, M.; Zava, O.; Dyson, P.J. Cellular uptake and subcellular distribution of ruthenium-based metallodrugs under clinical investigation versus cisplatin. Metallomics 2011, 3, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.A.; Flocke, L.S.; Hejl, M.; Roller, A.; Klose, M.H.M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Introducing the 4-phenyl-1,2,3-triazole moiety as a versatile scaffold for the development of cytotoxic ruthenium(II) and osmium(II) arene cyclometalates. Inorg. Chem. 2017, 56, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Endo, K.; Moriyama, H.; Tanaka, Y.; Alnemrii, E.S.; Slapak, C.A.; Teicher, B.; Kufe, D.; Datta, R. Abrogation of mitochondrial cytochrome c release and caspase-3 activation in acquired multidrug resistance. J. Biol. Chem. 1998, 273, 16647–16650. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kundu, S.; Bhattacharyya, A.; Hartinger, C.G.; Dyson, P.J. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J. Biol. Inorg. Chem. 2008, 13, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.H.; Romero-Canelón, I.; Fu, Y.; Shnyder, S.D.; Sadler, P.J. Potent organometallic osmium compounds induce mitochondria-mediated apoptosis and S-phase cell cycle arrest in A549 non-small cell lung cancer cells. Metallomics 2014, 6, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Rego, A.C.; Vesce, S.; Nicholls, D.G. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death Differ. 2001, 8, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 2006, 1757, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Tönnemann, J.; Risse, J.; Grote, Z.; Scopelliti, R.; Severin, K. Efficient and rapid synthesis of chlorido-bridged half-sandwich complexes of ruthenium, rhodium, and iridium by microwave heating. Eur. J. Inorg. Chem. 2013, 4558–4562. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Sanchez-Cano, C.; Clarkson, G.J.; Soni, R.; Wills, M.; Sadler, P.J. Easy to synthesize, robust organo-osmium asymmetric transfer hydrogenation catalysts. Chem. Eur. J. 2015, 21, 8043–8046. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Ru-Cl, Os-Cl, Ru-dca and Os-dca are available from the authors. |






| ng/106 Cells | fmol/106 Cells | |
|---|---|---|
| Ru-Cl | 26.5 ± 0.7 | 262.2 ± 7.0 |
| Ru-dca | 37.0 ± 1.4 | 366.1 ± 14.0 |
| Os-Cl | 33.5 ± 1.4 | 174.3 ± 7.4 |
| Os-dca | 52.5 ± 1.2 | 273.1 ± 6.4 |
| Sub-G1 | G0/G1 | S | G2/M | |
|---|---|---|---|---|
| Ru-Cl | 0.7 ± 0.2 | 62.4 ± 1.8 | 13.1 ± 2.8 | 23.2 ± 2.9 |
| Ru-dca | 16.8 ± 1.9 | 37.0 ± 2.7 | 24.3 ± 3.3 | 20.9 ± 1.1 |
| Os-Cl | 0.8 ± 0.3 | 59.0 ± 2.3 | 14.5 ± 0.9 | 25.1 ± 1.5 |
| Os-dca | 0.7 ± 0.2 | 62.9 ± 1.3 | 13.6 ± 4.7 | 22.2 ± 4.5 |
| Cisplatin | 8.0 ± 0.9 | 26.1 ± 2.1 | 30.9 ± 4.9 | 34.5 ± 3.5 |
| Control | 0.8 ± 0.3 | 69.2 ± 2.2 | 15.7 ± 1.4 | 13.9 ± 1.1 |
| % of Cells Showing High PE-Channel Fluorescence | |
|---|---|
| Ru-Cl | 94.0 ± 1.4 |
| Ru-dca | 89.3 ± 1.4 |
| Os-Cl | 95.2 ± 0.7 |
| Os-dca | 93.2 ± 2.3 |
| Cisplatin | 88.8 ± 2.6 |
| Positive control | 38.6 ± 1.7 |
| Negative control | 99.1 ± 0.3 |
| % of Cells Showing Shift in FITC-Channel Fluorescence | |
|---|---|
| Ru-Cl | 53.0 ± 5.7 |
| Ru-dca | 94.6 ± 0.6 |
| Os-Cl | 16.5 ± 4.1 |
| Os-dca | 39.9 ± 4.6 |
| Staurosporine | 47.1 ± 6.1 |
| Cisplatin | 53.0 ± 6.0 |
| Positive control | 99.3 ± 0.1 |
| Negative control | 12.3 ± 2.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štarha, P.; Trávníček, Z.; Vančo, J.; Dvořák, Z. Half-Sandwich Ru(II) and Os(II) Bathophenanthroline Complexes Containing a Releasable Dichloroacetato Ligand. Molecules 2018, 23, 420. https://doi.org/10.3390/molecules23020420
Štarha P, Trávníček Z, Vančo J, Dvořák Z. Half-Sandwich Ru(II) and Os(II) Bathophenanthroline Complexes Containing a Releasable Dichloroacetato Ligand. Molecules. 2018; 23(2):420. https://doi.org/10.3390/molecules23020420
Chicago/Turabian StyleŠtarha, Pavel, Zdeněk Trávníček, Ján Vančo, and Zdeněk Dvořák. 2018. "Half-Sandwich Ru(II) and Os(II) Bathophenanthroline Complexes Containing a Releasable Dichloroacetato Ligand" Molecules 23, no. 2: 420. https://doi.org/10.3390/molecules23020420
APA StyleŠtarha, P., Trávníček, Z., Vančo, J., & Dvořák, Z. (2018). Half-Sandwich Ru(II) and Os(II) Bathophenanthroline Complexes Containing a Releasable Dichloroacetato Ligand. Molecules, 23(2), 420. https://doi.org/10.3390/molecules23020420