In Silico-Based Repositioning of Phosphinothricin as a Novel Technetium-99m Imaging Probe with Potential Anti-Cancer Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Docking
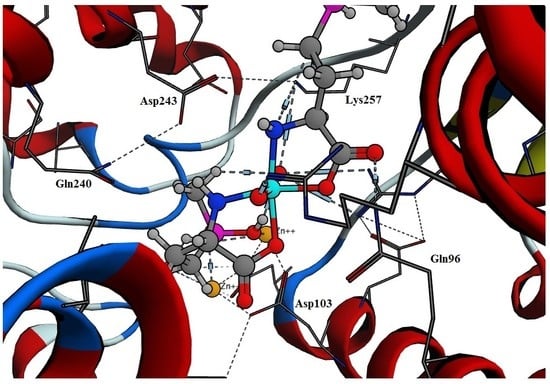
2.2. Docking of Tc-Phosphinothricin Complex
2.3. Molecular Dynamics
2.4. Molecular Dynamics Simulations of Tc-Phosphinothricin and Tc-Alendronate Complexes
2.5. Radiolabeling of Phosphinothricin.
2.5.1. Effect of Phosphinothricin Content
2.5.2. Effect of SnCl2·2H2O Content
2.5.3. Effect of pH of the Reaction Medium
2.5.4. Effect of Reaction Time
2.5.5. In Vitro Stability of 99mTc-phosphinothricin Complex
2.5.6. Biological Distribution Study
2.6. In Vitro Evaluation of the Anti-Cancer Activity
2.7. Bone Seeking, Imaging Probe and Anti-Cancer Activities
3. Material and Methods
3.1. Materials
3.2. Molecular Docking of the Phosphinothricin
3.3. Molecular Dynamics Simulations
3.4. Preparation of 99mTc-Phosphinothricin (99mTc-AHPB) Complex
3.4.1. Labeling Procedure
3.4.2. Radiochemical Yield of 99mTc-Phosphinothricin Complex
3.4.3. In Vitro Stability of 99mTc-Phosphinothricin Complex
3.4.4. Biodistribution Study
3.4.5. In Vitro Evaluation of the Anticancer Activity Using a Viability Assay
4. Conclusions
Acknowledgment
Conflicts of Interest
References
- Mitterhauser, M.; Toegel, S. What to Consider in the Development of New Bone Seekers: Mechanistic and Tracer-Related Aspects. Nucl. Med. Biol. 2008, 35, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Mitterhauser, M.; Toegel, S. Radiopharmaceutical Considerations on Bone Seeker Uptake: Should We Learn from Therapeutical Targets of Bisphosphonates? Nucl. Med. Biol. 2011, 38, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Jones, A.L. Comparison of 99mTc-labeled Phosphate and Phosphonate Agents for Skeletal Imaging. Semin. Nucl. Med. 1976, 6, 19–31. [Google Scholar] [CrossRef]
- Marty, R.; Denney, J.D.; McKamey, M.R.; Rowley, M.J. Comparison of 85Sr, 87mSr, 18F, and 99mTc-Labeled Phosphates for Bone Scanning. CRC Crit. Rev. Clin. Radiol. Nuc. Med. 1975, 6, 403–423. [Google Scholar]
- Tandon, V.R.; Sharma, S.; Mahajan, A. Bisphosphonate Drug Holidays: Can We Recommend Currently? J. Mid-Life Health 2014, 5, 111–114. [Google Scholar]
- Russell, R.G. Bisphosphonates: The First 40 Years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Mitterhauser, M.; Toegel, S. An in Vitro Model for the Comparative Evaluation of Bone Seeking Pharmaceuticals. Altex 2008, 25, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Motaleb, M.A.; Sakr, T.M. Synthesis and preclinical phramecological evaluation of 99mTc-TEDP as a novel bone imaging agent. J. Labell. Compd. Radiopharm. 2011, 54, 705–710. [Google Scholar] [CrossRef]
- Anderson, P.M.; Subbiah, V.; Rohren, E. Bone-Seeking Radiopharmaceuticals as Targeted Agents of Osteosarcoma: Samarium-153-EDTMP and Radium-223. Adv. Exp. Med. Biol. 2014, 804, 291–304. [Google Scholar] [PubMed]
- Sara, A.; Holstein, R.J.H. Inhibition of Farnesyl and Geranylgeranyl Diphosphate Synthases. Enzymes 2011, 30, 301–319. [Google Scholar]
- Santini, D.; Stumbo, L.; Spoto, C.; D’Onofrio, L.; Pantano, F.; Iuliani, M.; Fioramonti, M.; Zoccoli, A.; Ribelli, G.; Virzi, V.; et al. Bisphosphonates as Anticancer Agents in Early Breast Cancer: Preclinical and Clinical Evidence. Breast Cancer Res. 2015, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Clezardin, P. Mechanisms of Action of Bisphosphonates in Oncology: A Scientific Concept Evolving from Antiresorptive to Anticancer Activities. Bonekey Rep. 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, S.G.; Pirianov, G.; Mansi, J.L.; Arnett, T.R.; Colston, K.W. Bisphosphonates Induce Apoptosis in Human Breast Cancer Cell Lines. Br. J. Cancer 2000, 82, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Stachnik, A.; Yuen, T.; Iqbal, J.; Sgobba, M.; Gupta, Y.; Lu, P.; Colaianni, G.; Ji, Y.; Zhu, L.-L.; Kim, S.-M.; et al. Repurposing of Bisphosphonates for the Prevention and Therapy of Nonsmall Cell Lung and Breast Cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 17995–18000. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.R.; Lopez-Siles, F.J.; Cardenas, J. Resistance to Phosphinothricin (Glufosinate) and Its Utilization as a Nitrogen Source by Chlamydomonas Reinhardtii. Appl. Environ. Microbiol. 1996, 62, 3834–3839. [Google Scholar] [PubMed]
- Hoerlein, G. Glufosinate (phosphinothricin), a Natural Amino Acid with Unexpected Herbicidal Properties. Rev. Environ. Contam. Toxicol. 1994, 138, 73–145. [Google Scholar] [PubMed]
- Hack, R.; Ebert, E.; Ehling, G.; Leist, K.H. Glufosinate Ammonium—Some Aspects of its Mode of Action in Mammals. Food Chem. Toxicol. 1994, 32, 461–470. [Google Scholar] [CrossRef]
- Sakr, T.M.; Moustapha, M.E.; Motaleb, M.A. 99mTc-nebivolol as a Novel Heart Imaging Radiopharmaceutical for Myocardial Infarction Assessment. J. Radioanal. Nucl. Chem. 2013, 295, 1511–1516. [Google Scholar] [CrossRef]
- Srivastava, S.C.; Richards, P. Technetium-labled compounds. In Radiotracers for Medical Applications, CRC Series in Radiotracers in Bioliology and Medicine, 1st ed.; Rayudu, G.V.S., Ed.; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Wardell, J.L. Tin: Inorganic chemistry. In Encyclopedia of Inorganic Chemistry; King, R.B., Ed.; Wiley: New York, NY, USA, 1994; Volume 8. [Google Scholar]
- Saha, G.B. Fundamentals of Nuclear Pharmacy, 5th ed.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Kowalsky, R.J.; Falen, S.W. Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine, 3rd ed.; American Pharmacists Association: Washington, DC, USA, 2011. [Google Scholar]
- Noronha, O.P.D.; Venkateswarlu, K.S. On the Nature of Technetium Compounds. Eur. J. Nucl. Med. 1981, 6, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Huberty, J.P.; Hattner, R.S.; Powell, M.R. A 99mTc-Pyrophosphate Kit: A Convenient, Economical, and High-Quality Skeletal-Imaging Agent. J. Nucl. Med. 1974, 15, 124–126. [Google Scholar] [PubMed]
- Amin, A.M.; El-Azony, K.M.; Ibrahim, I.T. Application of 99Mo/99mTc Alumina Generator in the Labeling of Metoprolol for Diagnostic Purposes. J. Labell. Compd. Radiopharm. 2009, 52, 467–472. [Google Scholar] [CrossRef]
- Operating Environment MOE. Available online: https://www.chemcomp.com/Research-MOE_Citations.htm (accessed on 15 August 2016).
- Rondeau, J.M.; Bitsch, F.; Bourgier, E.; Geiser, M.; Hemmig, R.; Kroemer, M.; Lehmann, S.; Ramage, P.; Rieffel, S.; Strauss, A.; et al. Structural Basis for the Exceptional in vivo Efficacy of Bisphosphonate Drugs. ChemMedChem 2006, 1, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Essa, B.M.; Sakr, T.M.; Khedr, M.A.; El-Essawy, F.A.; El-Mohty, A.A. 99mTc-amitrole as a Novel Selective Imaging Probe for Solid Tumor: In Silico and Preclinical pharmacological Study. Eur. J. Pharm. Sci. 2015, 76, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In vitro preparation, optimization and in vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Rashed, H.; Marzook, F.; Farag, H. 99mTc-Zolmitriptan: Radiolabeling, Molecular Modeling, Biodistribution and Gamma Scintigraphy as a Hopeful Radiopharmaceutical for Lung Nuclear Imaging. Radiol. Med. 2016, 121, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Sakr, T.M.; Ibrahim, A.B.; Fasih, T.W.; Rashed, H.M. Preparation and Biological Profile of 99mTc-Lidocaine as a Cardioselective Imaging Agent Using 99mTc Eluted from 99Mo/99mTc Generator based on Al-Mo Gel. J. Radioanal. Nucl. Chem. 2017, 314, 1309–1317. [Google Scholar] [CrossRef]
- Konan, Y.N.; Cerny, R.; Favet, J.; Berton, M.; Gurny, R.; Allemann, E. Preparation and characterization of sterile sub-200 nm meso-tetra(4-hydroxylphenyl)porphyrin-loaded nanoparticles for photodynamic therapy. Eur. J. Pharm. Biopharm. 2003, 55, 115–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeong Lee, H.; Boado, R.J.; Pardridge, W.M. Receptor-Mediated Delivery of an Antisense Gene to Human Brain Cancer Cells. J Gene Med. 2002, 4, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Sakr, T.M.; El-Safoury, D.M.; Awad, G.A.; Motaleb, M.A. Biodistribution of 99mTc-Sunitinib as a Potential Radiotracer for Tumor Hypoxia Imaging. J. Labell. Compd. Radiopharm. 2013, 56, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.A.; Abdelmalak, N.S.; Naguib, M.J.; Rashed, H.M.; Ibrahim, A.B. Intranasal brain-targeted clonazepam polymeric micelles for immediate control of status epilepticus: In vitro optimization, ex vivo determination of cytotoxicity, in vivo biodistribution and pharmacodynamics studies. Drug Deliv. 2016, 23, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.O.; Nissan, Y.M.; El-Malah, A.A.; Ahmed, W.A.; Ibrahim, D.M.; Sakr, T.M.; Motaleb, M.A. Design, Synthesis and Bilogical Evaluation of Some Novel Sulfonamide Serivatives as Apoptotic Agents. Eur. J. Med. Chem. 2017, 135, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Al-wabli, R.I.; Sakr, T.M.; Khedr, M.A.; Adli, A.S.A.; Motaleb, M.A.; Zaghary, W.A. Platelet-12 Lipoxygenase Targeting via Newly Synthesized Curcumin Derivative Raiolabeled with Technetium-99m. Chem. Cent. J. 2016, 10, 73. [Google Scholar] [CrossRef] [PubMed]















| Compound | ΔG (kcal/mol) | Affinity pki | ΔG (Rescoring) (kcal/mol) |
|---|---|---|---|
| Phosphinothricin | −25.65 | 37.54 | −24.75 |
| Alendronate | −27.61 | 40.54 | −26.55 |
| Tc-phosphinothricin complex | −10.12 | 21.74 | −9.10 |
| Tc-Alendronate complex | −8.95 | 22.85 | −7.65 |
| Organ/Fluid | Time | ||||
|---|---|---|---|---|---|
| 10 min | 25 min | 1 h | 1.5 h | 2 h | |
| Blood | 41.51 ± 2.5 | 5.16 ± 1.2 | 4.76 ± 0.94 | 4.69 ± 1.09 | 4.68 ± 1.1 |
| Kidneys | 0.81 ± 0.2 | 10.04 ± 1.9 | 5.39 ± 1.05 | 4.30 ± 0.5 | 7.20 ± 0.8 |
| Liver | 1.44 ± 0.6 | 3.10 ± 0.9 | 13.55 ± 1.3 | 13.33 ± 0.93 | 15.23 ± 1.3 |
| Spleen | 0.32 ± 0.02 | 0.18 ± 0.2 | 1.49 ± 0.24 | 1.00 ± 0.02 | 0.19 ± 0.07 |
| Intestine | 2.21 ± 0.31 | 6.99 ± 0.46 | 6.26 ± 0.64 | 14.54 ± 2.01 | 34.12 ± 1.88 |
| Stomach | 4.80 ± 0.15 | 2.31 ± 0.52 | 1.33 ± 0.06 | 2.07 ± 0.5 | 4.54 ± 0.7 |
| Lungs | 2.37 ± 0.09 | 5.49 ± 0.33 | 5.45 ± 0.7 | 1.86 ± 0.1 | 2.23 ± 0.21 |
| Heart | 0.34 ± 0.07 | 3.24 ± 0.41 | 0.77 ± 0.03 | 1.73 ± 0.06 | 0.54 ± 0.08 |
| Muscle | 10.07 ± 1.1 | 9.93 ± 2.3 | 9.01 ± 0.29 | 3.83 ± 0.86 | 8.54 ± 1.1 |
| Bone | 36.13 ± 1.5 | 53.56 ± 3.1 | 51.99 ± 2.4 | 52.62 ± 2.6 | 22.73 ± 1.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakr, T.M.; Khedr, M.A.; Rashed, H.M.; Mohamed, M.E. In Silico-Based Repositioning of Phosphinothricin as a Novel Technetium-99m Imaging Probe with Potential Anti-Cancer Activity. Molecules 2018, 23, 496. https://doi.org/10.3390/molecules23020496
Sakr TM, Khedr MA, Rashed HM, Mohamed ME. In Silico-Based Repositioning of Phosphinothricin as a Novel Technetium-99m Imaging Probe with Potential Anti-Cancer Activity. Molecules. 2018; 23(2):496. https://doi.org/10.3390/molecules23020496
Chicago/Turabian StyleSakr, Tamer M., Mohammed A. Khedr, Hassan M. Rashed, and Maged E. Mohamed. 2018. "In Silico-Based Repositioning of Phosphinothricin as a Novel Technetium-99m Imaging Probe with Potential Anti-Cancer Activity" Molecules 23, no. 2: 496. https://doi.org/10.3390/molecules23020496
APA StyleSakr, T. M., Khedr, M. A., Rashed, H. M., & Mohamed, M. E. (2018). In Silico-Based Repositioning of Phosphinothricin as a Novel Technetium-99m Imaging Probe with Potential Anti-Cancer Activity. Molecules, 23(2), 496. https://doi.org/10.3390/molecules23020496