Determination of Propionylbrassinolide and Its Impurities by High-Performance Liquid Chromatography with Evaporative Light Scattering Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Identification of Impurities
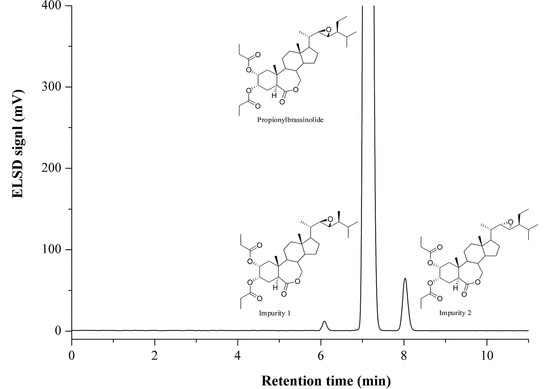
2.2. Optimization of Chromatographic Conditions
2.3. Calibration and Validation
2.4. LC-MS Analysis
2.5. Method Application
3. Experimental
3.1. Reagents and Materials
3.2. Semi-Preparative HPLC Conditions
3.3. HPLC-ELSD Analysis
3.4. Calibration
3.5. Method Validation
3.6. LC-MS Analysis
3.7. NMR Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mandava, N.B. Plant growth-promoting brassinosteroids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 23–52. [Google Scholar] [CrossRef]
- Bajguz, A.; Tretyn, A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 2003, 62, 1027–1046. [Google Scholar] [CrossRef]
- Zullo, M.A.T.; Adam, G. Brassinosteroid phytohormones-structure, bioactivity and applications. Braz. J. Plant Physiol. 2002, 14, 143–181. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Sun, X.; Ding, T.; Lei, B.; Zhang, C. Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 2017, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, X.; Yu, G.; Wang, J.; Wu, J.; Wang, M.; Yang, Y.; Shi, K.; Yu, Y.; Chen, Z.; et al. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci. Rep. 2015, 5, 9018. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Takatsuto, S.; Kamuro, Y.; Watanabe, T.; Kuriyama, H. Brassinosteroid Derivative and Plant Growth Regulator Using the Same. U.S. Patent US5763366A, 9 June 1998. [Google Scholar]
- Kanwar, M.K.; Bajguz, A.; Zhou, J.; Bhardwaj, R. Analysis of Brassinosteroids in Plants. J. Plant Growth Regul. 2017, 32, 1002–1030. [Google Scholar] [CrossRef]
- Pan, J.; Tan, W.; Li, G.; Hu, Y. Progress in the analysis of brassinosteroids. Chin. J. Chromatogr. 2011, 29, 105–110. [Google Scholar] [CrossRef]
- Gamoh, K.; Kitsuwa, T.; Takatsuto, S.; Fujimoto, Y.; Ikekawa, N. Determination of trace brassinosteroids by high performance liquid chromatography. Anal. Sci. 1988, 4, 533–535. [Google Scholar] [CrossRef]
- Gamoh, K.; Omote, K.; Okamoto, N.; Takatsuto, S. High-performance liquid chromatography of brassinosteroids in plants with derivatization using 9-phenanthreneboronic acid. J. Chromatogr. 1989, 469, 424–428. [Google Scholar] [CrossRef]
- Gamoh, K.; Sawamoto, H.; Takatsuto, S.; Watabe, Y.; Arimoto, H. Ferroceneboronic acid as a derivatization reagent for the determination of brassinosteroids by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. 1990, 515, 227–231. [Google Scholar] [CrossRef]
- Gamoh, K.; Okamoto, N.; Takatsuto, S.; Tejima, I. Determination of traces of natural brassinosteroids as dansylaminophenylboronates by liquid chromatography with fluorimetric detection. Anal. Chim. Acta 1990, 22, 101–105. [Google Scholar] [CrossRef]
- Huo, F.; Wang, X.; Han, Y.; Bai, Y.; Zhang, W.; Yuan, H.; Liu, H. A new derivatization approach for the rapid and sensitive analysis of brassinosteroids by using ultra high performance liquid chromatography-electrospray ionization triple quadrupole mass spectrometry. Talanta 2012, 99, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Wu, D.; Duan, C.; Guan, Y. Ultrasensitive quantification of endogenous brassinosteroids in milligram fresh plant with a quaternary ammonium derivatization reagent by pipette-tip solid-phase extraction coupled with ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1456, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J.; Wang, Y.L.; Liu, P.; Feng, Y.Q. 4-Phenylaminomethyl-benzeneboric acid modified tip extraction for determination of brassinosteroids in plant tissues by stable isotope labeling-liquid chromatography-mass spectrometry. Anal. Chem. 2016, 88, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Mao, L.J.; Guo, N.; Yu, L.; Feng, Y.Q. Determination of endogenous brassinosteroids using sequential magnetic solid phase extraction followed by in situ derivatization/desorption method coupled with liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1446, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.G.; Dong, F.S.; Hu, H.; Zheng, Y.Q. Residue analysis of propionylbrassinolide in fruit and vegetables by GC-MS. Chromatographia 2009, 69, 1453–1456. [Google Scholar] [CrossRef]
- Megoulas, N.C.; Koupparis, M.M.A. Twenty years of evaporative light scattering detection. Crit. Rev. Anal. Chem. 2005, 35, 301–316. [Google Scholar] [CrossRef]
- Lucena, R.; Cárdenas, S.; Valcárcel, M. Evaporative light scattering detection: trends in its analytical uses. Anal Bioanal. Chem. 2007, 388, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Dvorackova, E.; Snoblova, M.; Hrdlicka, P. Carbohydrate analysis: From sample preparation to HPLC on different stationary phases coupled with evaporative light-scattering detection. J. Sep. Sci. 2014, 37, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Condezo-Hoyos, L.; Pérez-López, E.; Rupérez, P. Improved evaporative light scattering detection for carbohydrate analysis. Food Chem. 2015, 180, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Song, X.; Zhang, M.; Li, E.; Gao, F.; He, L. Simultaneous determination of aminoglycoside antibiotics in feeds using high performance liquid chromatography with evaporative light scattering detection. RSC Adv. 2017, 7, 1251–1259. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Zhang, R.; Jiang, T.; Lv, Z. Determination of the triterpene glycosides in sea cucumbers by liquid chromatography with evaporative light scattering and mass spectrometry detection. J. Sep. Sci. 2015, 38, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, S.; Yang, F.; Li, P.; Wang, Y. Simultaneous determination of saponins and fatty acids in Ziziphus jujube (Suanzaoren) by high-performance liquid chromatography evaporative light scattering detection and pressurized liquid extraction. J. Chromatogr. A 2006, 1108, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Donot, F.; Cazals, G.; Gunata, Z.; Egron, D.; Malinge, J.; Strub, C.; Fontana, A.; Schorr-Galindo, S. Analysis of neutral lipids from microalgae by HPLC-ELSD and APCI-MS/MS. J. Chromatogr. B 2013, 942–943, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Z.; Zhai, X.; Sui, Z.; Wang, D.; Li, Q.; Bi, K.; He, B.; Wang, T. Simultaneous quantitative determination of 13 active components in the traditional Chinese medicinal preparation Suanzaoren oral liquid by HPLC coupled with diode array detection and evaporative light scattering detection. J. Sep. Sci. 2017, 40, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Escrig-Domenéch, A.; Simó-Alfonso, E.F.; Ramis-Ramos, G. Determination of the four major surfactant classes in cleaning products by reversed-phase liquid chromatography using serially connected UV and evaporative light-scattering detection. Anal. Chim. Acta 2016, 932, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, J.; Li, X.; Wang, D.; Huang, Q. Determination of brassinolide analogs by high-performance liquid chromatography with evaporative light scattering detection. Anal. Lett. 2015, 48, 5–585. [Google Scholar] [CrossRef]
- Konstantinova, O.V.; Antonchick, A.P.; Oldham, N.J.; Zhabinskii, V.N.; Khripach, V.A.; Schneider, B. Analysis of underivatized brassinosteroids by HPLC/APCI-MS. Occurrence of 3-epibrassinolide in Arabidopsis thaliana. Collect. Czech. Chem. Commun. 2011, 66, 1729–1734. [Google Scholar] [CrossRef]
- Swaczynová, J.; Novák, O.; Hauserová, E.; Fuksová, K.; Šíša, M.; Kohout, L.; Strnad, M. New techniques for the estimation of naturally occurring brassinosteroids. J. Plant Growth Regul. 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Sondhi, N.; Bhardwaj, R.; Kaur, S.; Kumar, N.; Singh, B. Isolation of 24-epibrassinolide from leaves of Aegle marmelos and evaluation of its antigenotoxicity employing Allium cepa chromosomal aberration assay. Plant Growth Regul. 2008, 54, 217–224. [Google Scholar] [CrossRef]
- Huo, F.; Bai, Y.; Liu, H. Fragmentation investigation of brassinosteroid compounds by ion trap and quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Tarkowská, D.; Novák, O.; Oklestkova, J.; Strnad, M. The determination of 22 natural brassinosteroids in a minute sample of plant tissue by UHPLC–ESI–MS/MS. Anal. Bioanal. Chem. 2016, 408, 6799–6812. [Google Scholar] [CrossRef] [PubMed]
- European Commission Directorate General Health and Consumer Protection. Technical Material and Preparations: Guidance for Generating and Reporting Methods of Analysis in Support of Pre-and Post-Registration Data Requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414, SANCO/3030/99 rev.4, 11/07/00. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwjz6-39_cTZAhXLfLwKHWnDAbMQFggqMAA&url=https%3A%2F%2Fec.europa.eu%2Ffood%2Fsites%2Ffood%2Ffiles%2Fplant%2Fdocs%2Fpesticides_ppp_app-proc_guide_phys-chem-ana_tech-mat-preps.pdf&usg=AOvVaw01Zd7ZdcCyiD44H2tgcS6- (accessed on 7 February 2018).
Sample Availability: Samples of the compounds propionylbrassinolide and its impurities 1 and 2 are available from the authors.article distributed under the terms and conditions of the Creative Commons Attribution. |






| Analyte | Linear Range (mg/L) | Calibration Curve a | R2 | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|
| Propionylbrassinolide | 4.3–543.3 | y = 1.5999x– 0.2860 | 0.9989 | 1.3 | 4.3 |
| Impurity 1 | 4.0–502.3 | y = 1.5912x – 0.3123 | 0.9997 | 1.2 | 4.0 |
| Impurity 2 | 4.2–539.3 | y = 1.6949x – 0.2952 | 0.9999 | 1.3 | 4.2 |
| Analyte | Repeatability (n = 7) | Intermediate Precision (n = 9) | ||
|---|---|---|---|---|
| Peak Area | Retention Time | Peak Area | Retention Time | |
| Propionylbrassinolide | 4.64 | 0.53 | 6.64 | 0.87 |
| Impurity 1 | 2.89 | 0.46 | 5.29 | 0.95 |
| Impurity 2 | 3.56 | 0.76 | 6.42 | 1.22 |
| Analyte | Mean Content (%, n = 7) a | %RSD | %RSDr |
|---|---|---|---|
| Propionylbrassinolide | 95.15 | 0.51 | 1.35 |
| Impurity 1 | 0.44 | 1.36 | 3.03 |
| Impurity 2 | 2.72 | 1.85 | 2.30 |
| Analyte | Original (mg/L) | Spiked (mg/L) | Observed (mg/L) | Recovery (%) | Mean (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Propionylbrassinolide | 90.10 | 33.95 | 124.23 | 100.53 | 99.07 | 1.32 |
| 125.30 | 33.95 | 158.58 | 98.03 | |||
| 170.28 | 33.95 | 203.77 | 98.65 | |||
| Impurity 1 | 3.81 | 3.93 | 7.26 | 87.79 | 92.03 | 4.70 |
| 5.06 | 3.93 | 8.85 | 96.44 | |||
| 7.25 | 3.93 | 10.86 | 91.86 | |||
| Impurity 2 | 30.85 | 16.85 | 46.88 | 95.13 | 95.96 | 1.45 |
| 39.24 | 16.85 | 55.28 | 95.19 | |||
| 52.21 | 16.85 | 68.65 | 97.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Zhang, H.; Zhang, H.; Yang, L.; Wu, M.; Zhou, P.; Huang, Q. Determination of Propionylbrassinolide and Its Impurities by High-Performance Liquid Chromatography with Evaporative Light Scattering Detection. Molecules 2018, 23, 531. https://doi.org/10.3390/molecules23030531
Cao L, Zhang H, Zhang H, Yang L, Wu M, Zhou P, Huang Q. Determination of Propionylbrassinolide and Its Impurities by High-Performance Liquid Chromatography with Evaporative Light Scattering Detection. Molecules. 2018; 23(3):531. https://doi.org/10.3390/molecules23030531
Chicago/Turabian StyleCao, Lidong, Hong Zhang, Hongjun Zhang, Li Yang, Miaomiao Wu, Puguo Zhou, and Qiliang Huang. 2018. "Determination of Propionylbrassinolide and Its Impurities by High-Performance Liquid Chromatography with Evaporative Light Scattering Detection" Molecules 23, no. 3: 531. https://doi.org/10.3390/molecules23030531
APA StyleCao, L., Zhang, H., Zhang, H., Yang, L., Wu, M., Zhou, P., & Huang, Q. (2018). Determination of Propionylbrassinolide and Its Impurities by High-Performance Liquid Chromatography with Evaporative Light Scattering Detection. Molecules, 23(3), 531. https://doi.org/10.3390/molecules23030531