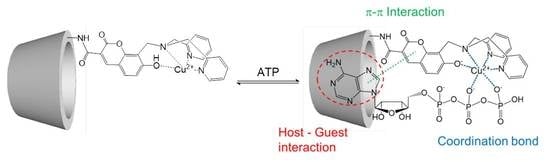
Development of Dipicolylamine-Modified Cyclodextrins for the Design of Selective Guest-Responsive Receptors for ATP
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 1α, 1β, and 1γ
2.2. Metal Ion Recognition by 1α, 1β, and 1γ
2.3. Phosphoric Acid Derivatives Recognitnion by 1α, 1β, and 1γ Metal Ion Complexes
2.4. 1D and 2D NMR Analyses
2.5. Suggested Supramolecular Conformation
3. Materials and Methods
3.1. Reagents
3.2. Apparatus
3.3. Metal Ion Recognition by 1α, 1β, and 1γ
3.4. Phosphoric Acid Derivatives Recognitnion by 1α, 1β, and 1γ Metal Ion Complexes
3.5. Calculation of Binding Constants of Cu·1α, Cu·1β, and Cu·1γ to ATP
3.6. NOESY Measurements of Zn·1β/ATP Complex
3.7. General Procedure for the Synthesis of 1
3.8. General Procedure for the Synthesis of 1α, 1β, and 1γ
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lehn, J.M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Hargrove, A.E.; Nieto, S.; Zhang, T.; Sessler, J.L.; Anslyn, E.V. Artificial Receptors for the Recognition of Phosphorylated Molecules. Chem. Rev. 2011, 111, 6603–6782. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Rao, C.P. Ion and Molecular Recognition by Lower Rim 1,3-Di-conjugates of Calix[4]arene as Receptors. Chem. Rev. 2011, 111, 4658–4702. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Hashimoto, T.; Hayashita, T. Supramolecular Functions of Cyclodextrin Complex Sensors for Sugar Recognition in Water. In Synergy in Supramolecular Chemistry; Nabeshima, T., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 235–246. ISBN 978-1-4665-9502-6. [Google Scholar]
- Wenz, G. Cyclodextrins as Building Blocks for Supramolecular Structures and Functional Units. Angew. Chem. Int. Ed. Engl. 1994, 33, 803–822. [Google Scholar] [CrossRef]
- Szente, L.; Szeman, J. Cyclodextrins in Analytical Chemistry: Host−Guest Type Molecular Recognition. Anal. Chem. 2013, 85, 8024–8030. [Google Scholar] [CrossRef] [PubMed]
- Kida, T.; Iwamoto, T.; Asahara, H.; Hinoue, T.; Akashi, M. Chiral Recognition and Kinetic Resolution of Aromatic Amines via Supramolecular Chiral Nanocapsules in Nonpolar Solvents. J. Am. Chem. Soc. 2013, 135, 3371–3374. [Google Scholar] [CrossRef] [PubMed]
- Klein, U.; Gimpl, G.; Fahrenholz, F. Alteration of the Myometrial Plasma Membrane Cholesterol Content with beta.-Cyclodextrin Modulates the Binding Affinity of the Oxytocin Receptor. Biochemistry 1995, 34, 13784–13793. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Harada, A. Chemical Sensors Based on Cyclodextrin Derivatives. Sensors 2008, 8, 4961–4982. [Google Scholar] [CrossRef] [PubMed]
- Hapiot, F.; Bricout, H.; Tilloy, S.; Monflier, E. Functionalized Cyclodextrins as First and Second Coordination Sphere Ligands for Aqueous Organometallic Catalysis. Eur. J. Inorg. Chem. 2012, 10, 1571–1578. [Google Scholar] [CrossRef]
- Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Photoswitchable Supramolecular Hydrogels Formed by Cyclodextrins and Azobenzene Polymers. Angew. Chem. Int. Ed. 2010, 49, 7461–7464. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Yui, N. Lysosomal-specific Cholesterol Reduction by Biocleavable Polyrotaxanes for Ameliorating Niemann-Pick Type C Disease. Sci. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, Y.; Zhang, Y.H.; Xu, X.; Liu, Y. Supramolecular Assembly of Coronene Derivatives for Drug Delivery. Org. Lett. 2016, 18, 4542–4545. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J.; Guo, D.S. Novel Permethylated β-Cyclodextrin Derivatives Appended with Chromophores as Efficient Fluorescent Sensors for the Molecular Recognition of Bile Salts. J. Org. Chem. 2007, 72, 8227–8234. [Google Scholar] [CrossRef] [PubMed]
- Kumai, M.; Kozuka, S.; Samizo, M.; Hashimoto, T.; Suzuki, I.; Hayashita, T. Glucose Recognition by a Supramolecular Complex of Boronic Acid Fluorophore with Boronic Acid-Modified Cyclodextrin in Water. Anal. Sci. 2012, 28, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Zhao, M.; Li, Y.; Liu, G.F.; Ji, L.N.; Mao, Z.W. An anthracene-modified β-cyclodextrin that distinguishes adenosine phosphates fluorescently. Tetrahedron Lett. 2014, 55, 1802–1805. [Google Scholar] [CrossRef]
- Eliseev, A.V.; Schneider, H.J. Molecular Recognition of Nucleotides, Nucleosides, and Sugars by Aminocyclodextrins. J. Am. Chem. Soc. 1994, 116, 6081–6088. [Google Scholar] [CrossRef]
- Yuan, D.Q.; Izuka, A.; Fukudome, M.; Rekharsky, M.V.; Inoue, Y.; Fujita, K. Heptakis(6-deoxy-6-guanidino)-β-cyclodextrin: An artificial model for mitochondrial ADP/ATP carrier. Tetrahedron Lett. 2007, 48, 3479–3483. [Google Scholar] [CrossRef]
- Schwinte, P.; Darcy, R.; O’Keeffe, F. Ditopic binding of nucleotides by heptakis(6-hydroxyethylamino-6-deoxy)-β-cyclodextrin. J. Chem. Soc. Perkin Trans. 1998, 2, 805–808. [Google Scholar] [CrossRef]
- Vizitiu, D.; Thatcher, G.R.J. Binding of Phosphates to Aminocyclodextrin Biomimetics. J. Org. Chem. 1999, 64, 6235–6238. [Google Scholar] [CrossRef]
- Cotner, E.S.; Smith, P.J. Phosphotyrosine Binding by Ammonium- and Guanidinium-Modified Cyclodextrins. J. Org. Chem. 1998, 63, 1737–1739. [Google Scholar] [CrossRef]
- Hauser, S.L.; Johanson, E.W.; Green, H.P.; Smith, P.J. Aryl Phosphate Complexation by Cationic Cyclodextrins. An Enthalpic Advantage for Guanidinium over Ammonium and Unusual Enthalpy−Entropy Compensation. Org. Lett. 2000, 2, 3575–3578. [Google Scholar] [CrossRef] [PubMed]
- Mourtzis, N.; Eliadou, K.; Aggelidou, C.; Sophianopoulou, V.; Mavridis, I.M.; Yannakopoulou, K. Per(6-guanidino-6-deoxy)cyclodextrins: Synthesis, characterisation and binding behaviour toward selected small molecules and DNA. Org. Biomol. Chem. 2007, 5, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Aggelidou, C.; Mavridis, I.M.; Yannakopoulou, K. Binding of Nucleotides and Nucleosides to Per(6-guanidino-6-deoxy)cyclodextrins in Solution. Eur. J. Org. Chem. 2009, 2299–2305. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ojida, A.; Hamachi, I. Molecular recognition, fluorescence sensing, and biological assay of phosphate anion derivatives using artificial Zn(II)–Dpa complexes. Chem. Commun. 2009, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kurishita, Y.; Kojira, T.; Ojida, A.; Hamachi, I. Rational Design of FRET-Based Ratiometric Chemosensors for in Vitro and in Cell Fluorescence Analyses of Nucleoside Polyphosphates. J. Am. Chem. Soc. 2010, 132, 13290–13299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, M.S.; Luo, H.; Zhang, Q.; Guo, L.E.; Li, Z.; Jiang, Y.B. Aggregation-Switching Strategy for Promoting Fluorescent Sensing of Biologically Relevant Species: A Simple Near-Infrared Cyanine Dye Highly Sensitive and Selective for ATP. Anal. Chem. 2017, 89, 6210–6215. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Fujiwara, S.; Yamada, T.; Tsuchido, Y.; Hashimoto, T.; Hayashita, T. Design and Function of Supramolecular Recognition Systems Based on Guest-Targeting Probe-Modified Cyclodextrin Receptors for ATP. J. Org. Chem. 2017, 82, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.; Liu, X.; Jolliffe, K.A. Anion recognition and sensing with Zn(II)–dipicolylamine complexes. Chem. Soc. Rev. 2012, 41, 4928–4965. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Swamy, K.M.K.; Lee, K.M.; Jagdale, A.R.; Kim, Y.; Kim, S.J.; Yoo, K.H.; Yoon, J. Pyrophosphate selective fluorescent chemosensors based on coumarin–DPA–Cu(II) complexes. Chem. Commun. 2009, 7215–7217. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Takahashi, K. Molecular properties of mono guest-modified cyclodextrins on the secondary site. Supramol. Chem. 2011, 23, 156–159. [Google Scholar] [CrossRef]
- De Silva, A.P.; Moody, T.S.; Wright, G.D. Fluorescent PET (photoinduced electron transfer) sensors as potent analytical tools. Analyst 2009, 134, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Quang, D.T. Calixarene-Derived Fluorescent Probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar] [CrossRef] [PubMed]
- Santis, G.D.; Fabbrizzi, L.; Licchelli, M.; Mangano, C.; Sacchi, D. Redox Switching of Anthracene Fluorescence through the CuII/CuI Couple. Inorg. Chem. 1995, 34, 3581–3582. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, S.Y.; Hong, J.I. A Fluorescent Pyrophosphate Sensor with High Selectivity over ATP in Water. Angew. Chem. Int. Ed. 2004, 43, 4777–4780. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Singh, N.J.; Lim, J.; Pan, J.; Kim, H.N.; Park, S.; Kim, K.S.; Yoon, J. Unique Sandwich Stacking of Pyrene-Adenine-Pyrene for Selective and Ratiometric Fluorescent Sensing of ATP at Physiological pH. J. Am. Chem. Soc. 2009, 131, 15528–15533. [Google Scholar] [CrossRef] [PubMed]
- Wenska, G.; Paszyc, S. Coumarin–nucleotide base pairs. Ultraviolet absorption, fluorescence, and photochemical study. Can. J. Chem. 1984, 62, 2006–2010. [Google Scholar] [CrossRef]
- Roy, B.; Rao, A.S.; Ahn, K.H. Mononuclear Zn(II)- and Cu(II)-complexes of a hydroxynaphthalene-derived dipicolylamine: Fluorescent sensing behaviours toward pyrophosphate ions. Org. Biomol. Chem. 2011, 9, 7774–7779. [Google Scholar] [CrossRef] [PubMed]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Ojida, A.; Inoue, M.; Mito-oka, Y.; Tsutsumi, H.; Sada, K.; Hamachi, I. Effective Disruption of Phosphoprotein−Protein Surface Interaction Using Zn(II) Dipicolylamine-Based Artificial Receptors via Two-Point Interaction. J. Am. Chem. Soc. 2006, 128, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.W.; Lee, C.R.; Cho, S.H.; Song, M.R.; Cashel, M.; Choy, H.E.; Seok, Y.J.; Hong, J.I. Selective Fluorescent Chemosensor for the Bacterial Alarmone (p)ppGpp. J. Am. Chem. Soc. 2008, 130, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Ojida, A.; Sakamoto, T.; Inoue, M.; Fujishima, S.; Lippens, G.; Hamachi, I. Fluorescent BODIPY-Based Zn(II) Complex as a Molecular Probe for Selective Detection of Neurofibrillary Tangles in the Brains of Alzheimer’s Disease Patients. J. Am. Chem. Soc. 2009, 131, 6543–6548. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pham, D.T.; Kee, T.W.; Clafton, S.N.; Guo, X.; Clements, P.; Lincoln, S.F.; Prud’homme, R.K.; Easton, C.J. Aggregation and Host–Guest Interactions in Dansyl-Substituted Poly(acrylate)s in the Presence of β-Cyclodextrin and a β-Cyclodextrin Dimer in Aqueous Solution: A UV–Vis, Fluorescence, 1H NMR, and Rheological Study. Macromolecules 2011, 44, 9782–9791. [Google Scholar] [CrossRef]
- Wang, H.; Shao, N.; Qiao, S.; Cheng, Y. Host–Guest Chemistry of Dendrimer–Cyclodextrin Conjugates: Selective Encapsulations of Guests within Dendrimer or Cyclodextrin Cavities Revealed by NOE NMR Techniques. J. Phys. Chem. 2012, 116, 11217–11224. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |









| Compound | 1 | DCC | HOBt·H2O | 3-NH2-(𝛼, 𝛽, or γ)-CyD |
|---|---|---|---|---|
| 1α | 44 mg, 0.11 mmol | 24 mg, 0.11 mmol | 18 mg, 0.12 mmol | 70 mg, 0.072 mmol |
| 1β | 56 mg, 0.13 mmol | 28 mg, 0.14 mmol | 21 mg, 0.14 mmol | 102 mg, 0.090 mmol |
| 1γ | 42 mg, 0.10 mmol | 22 mg, 0.11 mmol | 16 mg, 0.10 mmol | 82 mg, 0.063 mmol |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Fujiwara, S.; Fujita, K.; Tsuchido, Y.; Hashimoto, T.; Hayashita, T. Development of Dipicolylamine-Modified Cyclodextrins for the Design of Selective Guest-Responsive Receptors for ATP. Molecules 2018, 23, 635. https://doi.org/10.3390/molecules23030635
Yamada T, Fujiwara S, Fujita K, Tsuchido Y, Hashimoto T, Hayashita T. Development of Dipicolylamine-Modified Cyclodextrins for the Design of Selective Guest-Responsive Receptors for ATP. Molecules. 2018; 23(3):635. https://doi.org/10.3390/molecules23030635
Chicago/Turabian StyleYamada, Tatsuru, Shoji Fujiwara, Kyohhei Fujita, Yuji Tsuchido, Takeshi Hashimoto, and Takashi Hayashita. 2018. "Development of Dipicolylamine-Modified Cyclodextrins for the Design of Selective Guest-Responsive Receptors for ATP" Molecules 23, no. 3: 635. https://doi.org/10.3390/molecules23030635
APA StyleYamada, T., Fujiwara, S., Fujita, K., Tsuchido, Y., Hashimoto, T., & Hayashita, T. (2018). Development of Dipicolylamine-Modified Cyclodextrins for the Design of Selective Guest-Responsive Receptors for ATP. Molecules, 23(3), 635. https://doi.org/10.3390/molecules23030635