Catalytic Annulation of Epoxides with Heterocumulenes by the Indium-Tin System
Abstract
:1. Introduction
2. Results
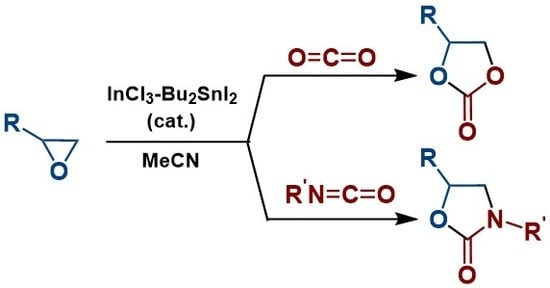
2.1. Synthesis of Cyclic Carbonates
2.2. Synthesis of 2-Oxazolidinones
3. Discussion
4. Materials and Methods
4.1. Analysis
4.2. General Procedure for Synthesis of Cyclic Carbonates 2a–f from Epoxides 1 with CO2
4.3. General Procedure for Synthesis of 2-Oxazolidinones 4 from Epoxides 1b–f with Isocyanates 3 and Oxazolidin-2-imine 5
4.4. Observation of Tin-Indium System by 119Sn NMR
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Behrens, C.H.; Sharpless, K.B. New transformation of 2,3-epoxy alcohols and related derivatives. Easy routes to homochiral substances. Aldrichim. Acta 1983, 16, 67–79. [Google Scholar] [CrossRef]
- Rao, A.S.; Paknikar, S.K.; Kirtane, J.G. Recent advances in the preparation and synthetic applications of oxiranes. Tetrahedron 1983, 39, 2323–3367. [Google Scholar] [CrossRef]
- Smith, J.G. Synthetically useful reactions of epoxides. Synthesis 1984, 1984, 629–656. [Google Scholar] [CrossRef]
- Bercaw, J.E.; Creutz, C.; Dinjus, E.; Dixon, D.A.; Domen, K.; DuBois, D.L.; Eckert, J.; Fujita, E.; Gibson, D.H.; Goddard, W.A.; et al. Catalysis research of relevance to carbon management: Progress, challenges, and opportunitie. Chem. Rev. 2001, 101, 953–996. [Google Scholar]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A. The contribution of the utilization option to reducing the CO2 atmospheric loading: research needed to overcome existing barriers for a full exploitation of the potential of the CO2 use. Catal. Today 2004, 98, 455–462. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. Aspects of carbon dioxide utilization. Catal. Today 2006, 115, 33–52. [Google Scholar] [CrossRef]
- Shaikh, A.-A.G.; Sivaram, S. Organic carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.H. Reactive applications of cyclic alkylene carbonates. Ind. Eng. Chem. Res. 2003, 42, 663–674. [Google Scholar] [CrossRef]
- Schaffner, B.; Holz, J.; Verevkin, S.P.; Borner, A. Organic carbonates as alternative solvents for palladium-catalyzed substitution reactions. ChemSusChem 2008, 1, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bayardon, J.; Holz, J.; Schaffner, B.; Andrushko, V.; Verevkin, S.; Preetz, A.; Borner, A. Propylene carbonate as a solvent for asymmetric hydrogenations. Angew. Chem. Int. Ed. 2007, 46, 5971–5974. [Google Scholar] [CrossRef] [PubMed]
- Lagowski, J.J. The Chemistry of Nonaqueous Solvents; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Inaba, M.; Siroma, Z.; Funabiki, A.; Ogumi, A.; Abe, T.; Mizutani, Y.; Asano, M. Electrochemical scanning tunneling microscopy observation of highly oriented pyrolytic graphite surface reactions in an ethylene carbonate-based electrolyte solution. Langmuir 1996, 12, 1535–1540. [Google Scholar] [CrossRef]
- Fukuoka, S.; Kawamura, M.; Komiya, K.; Tojo, M.; Hachiya, H.; Hasegawa, K.; Aminaka, M.; Okamoto, H.; Fukawa, I.; Konno, S. A novel non-phosgene polycarbonate production process using by-product CO2 as starting material. Green Chem. 2003, 5, 497–507. [Google Scholar] [CrossRef]
- Dyen, M.E.; Swern, D. 2-Oxazolidones. Chem. Rev. 1967, 67, 197–246. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S. Recent advances in isocyanate chemistry. Chem. Rev. 1972, 72, 457. [Google Scholar] [CrossRef]
- Shiozaki, M. Pharmacology of oxazolidinones in rat decerebrate rigidity, with reference to their glutamate blocking action. Gen. Pharm. 1988, 19, 163–169. [Google Scholar] [CrossRef]
- Barbachyn, M.R.; Ford, C.W. Oxazolidinone structure-activity relationships leading to linezolid. Angew. Chem. Int. Ed. 2003, 42, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Fan, H.; Xin, Q.; Chu, W.; Wang, H.; Huang, Y.; Chen, X.; Yang, Y. Solubility-driven optimization of (pyridin-3-yl) benzoxazinyl-oxazolidinones leading to a promising antibacterial agent. J. Med. Chem. 2013, 56, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Trstenjak, U.; Ilas, J.; Kikelj, D. Low molecular weight dual inhibitors of factor Xa and fibrinogen binding to GPIIb/IIIa with highly overlapped pharmacophores. Eur. J. Med. Chem. 2013, 64, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Ding, S.; Guo, B.; Zhou, Y.; Sun, P.; Wang, H.; Chu, W.; Gong, G.; Wang, Y.; Chen, X.; et al. Design, synthesis, and structure-activity and structure-pharmacokinetic relationship studies of novel [6,6,5] tricyclic fused oxazolidinones leading to the discovery of a potent, selective, and orally bioavailable FXa inhibitor. J. Med. Chem. 2014, 57, 7770–7791. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Orena, M.; Sandri, S.; Tomashini, C. An efficient synthesis of (R)-(+)- and (S)-(−)-propranolol from resolved 5-idomethyloxazo-lidin-2-ones. Tetrahedron 1987, 43, 2505–2512. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Dhar, T.G.M.; Chakraborty, T.K.; Gurjar, M.K. A stereospecific synthesis of (4R)-4-[(E)-2-butenyl]-4, N-dimethyl-L-threonine (MeBmt). Tetrahedron Lett. 1988, 29, 2069–2072. [Google Scholar] [CrossRef]
- Ager, D.J.; Prakash, I.; Schaad, D.R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996, 96, 835–876. [Google Scholar] [CrossRef] [PubMed]
- Roush, W.R.; James, R.A. Towards the synthesis of aureolic acid analogue conjugates: Synthesis and glycosidation reactions of 3-O-acetyl-4-azido-2,4,6-trideoxy-l-glucopyranose derivatives. Aust. J. Chem. 2002, 55, 141–146. [Google Scholar] [CrossRef]
- Aurelio, L.; Brownlee, R.T.C.; Hughes, A.B. Synthetic preparation of N-methyl-α-amino acids. Chem. Rev. 2004, 104, 5823–5846. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.A.; Wright, G.D. Streptogramins, oxazolidinones, and other inhibitors of bacterial protein synthesis. Chem. Rev. 2005, 105, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Birrell, J.A.; Jacobsen, E.N. A practical method for the synthesis of highly enantioenriched trans-1,2-amino alcohols. Org. Lett. 2013, 15, 2895–2897. [Google Scholar] [CrossRef] [PubMed]
- Laserna, V.; Guo, W.; Kleij, A.W. Aluminium-catalysed oxazolidinone synthesis and their conversion into functional non-symmetrical ureas. Adv. Synth. Catal. 2015, 357, 2849–2854. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Farajpour, B. Applications of oxazolidinones as chiral auxiliaries in the asymmetric alkylation reaction applied to total synthesis. RSC Adv. 2016, 6, 30498–30551. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W. Catalysts for the reactions of epoxides and carbon dioxide. Coord. Chem. Rev. 1996, 153, 155–174. [Google Scholar] [CrossRef]
- Sun, J.; Fujita, S.; Arai, M. Development in the green synthesis of cyclic carbonate from carbon dioxide using ionic liquids. J. Organomet. Chem. 2005, 690, 3490–3497. [Google Scholar] [CrossRef]
- Cintas, P. Synthetic organoindium chemistry: What makes indium so appealing? Synlett 1995, 1995, 1087–1096. [Google Scholar] [CrossRef]
- Shibata, I.; Mitani, I.; Imakuni, A.; Baba, A. Highly efficient synthesis of cyclic carbonates from epoxides catalyzed by indium tribromide system. Tetrahedron Lett. 2011, 52, 721–723. [Google Scholar] [CrossRef]
- Gulbins, K.; Hamann, M. Darstellung von oxazolidonen. Angew. Chem. 1958, 70, 705. [Google Scholar] [CrossRef]
- Gulbins, K.; Benzing, G.; Maysenholder, R.; Hamann, K. Synthese von substituierten oxazolidone-(2). Chem. Ber. 1960, 93, 1975–1982. [Google Scholar] [CrossRef]
- Irwin, W.J.; Wheeler, D.L. 3,5-Diphenyloxazolidin-2-one. J. Chem. Soc. C 1971, 66, 3166–3167. [Google Scholar] [CrossRef]
- Herweh, J.E.; Foglia, T.A.; Swern, D.J. Synthesis and nuclear magnetic resonance spectra of 2-oxazoli-dones. J. Org. Chem. 1968, 33, 4029–4033. [Google Scholar] [CrossRef]
- Herweh, J.E. Bis-2-oxazolidones-preparation and characterization. J. Heterocycl. Chem. 1968, 5, 687–690. [Google Scholar] [CrossRef]
- Herweh, J.E.; Kaufmann, W.J. 2-Oxazolidones via the lithium bromide catalyzed reaction of isocyanates with epoxides in hydrocarbon solvents. Tetrahedron Lett. 1971, 12, 809–812. [Google Scholar] [CrossRef]
- Aroua, L.; Baklouti, A. α,ω-Bis(oxazolidinone)polyoxyethylene via a lithium bromide–catalyzed reaction of oligoethylene glycol diglycidyl ethers with isocyanates. Synth. Commun. 2007, 37, 1935–1942. [Google Scholar] [CrossRef]
- Speranza, G.P.; Peppel, W.J. Preparation of substituted 2-oxazolidones from 1,2-epoxides and isocyanates. J. Org. Chem. 1958, 23, 1922–1924. [Google Scholar] [CrossRef]
- Weiner, M.L. Reaction of phenyl isocyanate with phenyl glycidyl ether. J. Org. Chem. 1961, 26, 951–952. [Google Scholar] [CrossRef]
- Toda, Y.; Gomyou, S.; Tanaka, S.; Komiyama, Y.; Kikuchi, A.; Suga, H. Tetraarylphosphonium salt-catalyzed synthesis of oxazolidinones from Isocyanates and Epoxides. Org. Lett. 2017, 19, 5786–5789. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Weinert, J. Umsetzung von epoxiden mit isocyanaten, II. Darstellung und charakterisierung von 2-oxazolidinonen. Eur. J. Org. Chem. 1979, 1979, 200–209. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Zhao, C.; Li, C.; Wu, X.; Chen, W.Z. A Facile and efficient synthesis of 3-aryloxazolidin-2-ones from isocyanates and epoxides promoted by MgI2 etherate. Synth. Commun. 2010, 40, 3654–3659. [Google Scholar] [CrossRef]
- Baba, A.; Fujiwara, M.; Matsuda, H. Unusual cycloaddition of oxiranes with isocyanates catalyzed by tetraphenylstibonium iodide; selective formation of 3,4-disubstituted oxazolidinones. Tetrahedron Lett. 1986, 27, 77–80. [Google Scholar] [CrossRef]
- Fujiwara, M.; Baba, A.; Matsuda, H. Selective α-cleavage cycloaddition of oxiranes with heterocumulenes catalyzed by tetraphenylstibonium iodide. J. Heterocycl. Chem. 1988, 25, 1351–1357. [Google Scholar] [CrossRef]
- Fujiwara, M.; Baba, A.; Matsuda, H. Mechanistic studies of tetraphenylstibonium iodide-catalyzed cycloaddition of oxiranes with heterocumulenes. Bull. Chem. Soc. Jpn. 1990, 63, 1069–1073. [Google Scholar] [CrossRef]
- Wu, X.; Mason, J.; North, M. Isocyanurate formation during oxazolidinone synthesis from epoxides and isocyanates catalysed by a chromium(salphen) complex. Chem. Eur. J. 2017, 23, 12937–12943. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Sudhakar, A.R. Cis Hydroxyamination equivalent. Application to the synthesis of (-)-acosamine. J. Am. Chem. Soc. 1987, 109, 3792–3794. [Google Scholar] [CrossRef]
- Shibata, I.; Baba, A.; Iwasaki, H.; Matsuda, H. Cycloaddition reaction of heterocumulenes with oxiranes catalyzed by organotin iodide-Lewis base complex. J. Org. Chem. 1986, 51, 2177–2184. [Google Scholar] [CrossRef]
- Fujiwara, M.; Baba, A.; Tomohisa, Y.; Matsuda, H. Cycloaddition reaction of 2,3-disubstituted oxiranes with isocyanates by highly activated catalyst; Ph4SbI–Bu3SnI. Chem. Lett. 1986, 15, 1963–1966. [Google Scholar] [CrossRef]
- Baba, A.; Seki, K.; Matsuda, H. Stereospecific cycloaddition of heterocumulenes to oxiranes catalyzed by organotin halide complexes. J. Heterocycl. Chem. 1990, 27, 1925–1930. [Google Scholar] [CrossRef]
- Yano, K.; Amishiro, N.; Baba, A.; Matsuda, H. Selective formation of α-cleavage cycloadduct of oxirane with heterocumulene promoted by high-coordinated trialkyltin complexes. Bull. Chem. Soc. Jpn. 1991, 64, 2661–2667. [Google Scholar] [CrossRef]
- Tsuzuki, R.; Ishikawa, K.; Kase, M. New reactions of organic isocyanates. I. Reaction with alkylene carbonates. J. Org. Chem. 1960, 25, 1009–1012. [Google Scholar] [CrossRef]
- Holecek, J.; Nádvorník, M.; Handlír, K.; Lycka, A. 13C and 119Sn NMR Study of some four- and five-coordinate triphenyltin(IV) compounds. J. Organomet. Chem. 1983, 241, 177–184. [Google Scholar] [CrossRef]
- Nádvorník, M.; Holecek, J.; Handlír, K.; Lycka, A. The 13C and 119Sn NMR spectra of some four- and five-coordinate tri-n-butyltin(IV) compounds. J. Organomet. Chem. 1984, 275, 43–51. [Google Scholar] [CrossRef]
- Bonini, C.; Righi, G. Regio- and chemoselective synthesis of halohydrins by cleavage of oxiranes with metal halides. Synthesis 1994, 1994, 225–238. [Google Scholar] [CrossRef]
- Backwell, J.E.; Young, M.W.; Sharpless, K.B. Vicinal acetoxychlorination of olefins by chromyl chloride in acetyl chloride. Tetrahedron Lett. 1977, 40, 3523–3526. [Google Scholar]
- Shibata, I.; Baba, A.; Matsuda, H. Regioselective ring cleavage of oxiranes catalyzed by organotin halide—Triphenylphosphine complex. Tetrahedron Lett. 1986, 27, 3021–3024. [Google Scholar] [CrossRef]
- Shibata, I.; Yoshimura, N.; Baba, A.; Matsuda, H. Remarkable dependency of the regioselectivity in the ring opening of α,β-epoxyketones upon tin halide-Lewis base complexes as catalysts. Tetrahedron Lett. 1992, 33, 7149–7152. [Google Scholar] [CrossRef]
- Baba, A.; Shibata, I. Dihaloindium hydride as a novel reducing agent. Chem. Rec. 2005, 5, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Sawada, A.; Shibata, I.; Baba, A. Indium hydride: A novel radical initiator in the reduction of organic halides with tributyltin hydride. Tetrahedron Lett. 2001, 42, 4661–4663. [Google Scholar] [CrossRef]
- Miyai, T.; Inoue, K.; Baba, A. Indoum triiodide (InI3)-catalyzed allylation of carbonyl compounds by allylic tins. Synlett 1997, 1997, 699–700. [Google Scholar] [CrossRef]
- Marshall, J.A.; Hinkle, K.W. Synthesis of anti-homoallylic alcohols and monoprotected 1,2-diols through InCl3-promoted addition of allylic stannanes to aldehydes. J. Org. Chem. 1995, 60, 1920–1921. [Google Scholar] [CrossRef]
- Li, X.-R.; Loh, T.-P. Indium trichloride-promoted tin-mediated carbonyl allylation in water: High simple diastereo- and diastereofacial selectivity. Tetrahedron Asymmetry 1996, 7, 1535–1538. [Google Scholar] [CrossRef]
- Baba, A.; Kishiki, H.; Shibata, I.; Matsuda, H. Reaction of tributyltin ω-haloalkoxides with isocyanates or carbodiimides. A possibility of the addition of a tin-oxygen bond across the carbon-oxygen group of isocyanate Organometallics 1984, 4, 1329–1333. [Google Scholar]
- Shibata, I.; Baba, A.; Matsuda, H. Formation of N-tributylstannyl-2-oxazolidone from (Bu3Sn)2O and 2-chloroethyl isocyanate. J. Chem. Soc. Chem. Commun. 1986, 1703–1704. [Google Scholar] [CrossRef]
- Shibata, I.; Nakamura, K.; Baba, A.; Matsuda, H. Formation of N-tributylstannyl heterocycle from bis(tributyltin) oxide and ω-haloalkyl isocyanate. One-pot convenient synthesis of 2-oxazolidinones and tetrahydro-2H-1,3-oxazin-2-one. Bull. Chem. Soc. Jpn. 1989, 62, 853–859. [Google Scholar] [CrossRef]
- Delmond, B.; Pommier, J.C.; Valade, J. Halogénoalcoxyétains: I. (halogéno-2 alcoy)tributylétains, synthéses et propriétés. application á la préparation d’époxydes. J. Organomet. Chem. 1972, 35, 91–104. [Google Scholar] [CrossRef]
- Delmond, B.; Pommier, J.C.; Valade, J. Halogénoalcoxyétains II. halogéno-3 alcoxytributylétains, synthéses et propriétés-application à la preéparation d’oxétannes et d’alcools stanniques. J. Organomet. Chem. 1973, 47, 337–350. [Google Scholar] [CrossRef]
- Suzuki, I.; Uji, Y.; Ieki, R.; Kanaya, S.; Tsunoi, S.; Shibata, I. Transition-metal-free reductive coupling of 1,3-butadienes with aldehydes catalyzed by dibutyliodotin hydride. Org. Lett. 2017, 19, 5392–5394. [Google Scholar] [CrossRef] [PubMed]
- Whiteoak, C.J.; Martin, E.; Belmonte, M.M.; Benet-Buchholz, J.; Kleij, A.W. An efficient iron catalyst for the synthesis of five- and six-membered organic carbonates under mild conditions. Adv. Synth. Catal. 2012, 354, 469–476. [Google Scholar] [CrossRef]
- Liu, X.; Cao, C.; Li, Y.; Guan, P.; Yang, L.; Shi, Y. Cycloaddition of CO2 to epoxides catalyzed by N-heterocyclic carbene (NHC)–ZnBr2 System under mild conditions. Synlett 2012, 43, 1343–1348. [Google Scholar] [CrossRef]
- Baba, A.; Shibata, I.; Matsuda, K.; Matsuda, H. The cycloaddition of isocyanates and carbodiimides to oxiranes catalyzed by organotin iodide-Lewis base complexes. Synthesis 1985, 1985, 1144–1146. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Entry | R | Cat. | Time (h) | Product | Yield 2 (%) b | |
|---|---|---|---|---|---|---|
| 1 | Me (1a) | InCl3 | 5 |  | 2a | trace |
| 2 | InCl3-Bu2SnI2 | 5 | 78 | |||
| 3 | Bu2SnI2 | 5 | trace | |||
| 4 | Et (1b) | InCl3-Bu2SnI2 | 8 |  | 2b | 85 |
| 5 | Ph (1c) | InCl3-Bu2SnI2 | 10 |  | 2c | 69 |
| 6 | CH2Cl (1d) | InCl3-Bu2SnI2 | 5 |  | 2d | 82 |
| 7 | CH2OPh (1e) | InCl3-Bu2SnI2 | 5 |  | 2e | 90 |
| 8 | CH2OMe (1f) | InCl3-Bu2SnI2 | 10 |  | 2f | 68 |

| Entry | R1 | R2 | Conditions | Product | Yield 3 (%) b | |
|---|---|---|---|---|---|---|
| 1 | Et (1b) | t-Bu (3a) | rt, 10 h |  | 4a | 79 |
| 2 | 7 c | |||||
| 3 | trace d | |||||
| 4 | CH2Cl (1d) | 60 °C, 3 h |  | 4b | 79 | |
| 5 | CH2OPh (1e) | 60 °C, 3 h |  | 4c | 90 | |
| 6 | CH2OMe (1f) | 60 °C, 3 h |  | 4d | 99 | |
| 7 | Et (1b) | n-Bu (3b) | 60 °C, 7 h |  | 4e | 49 |
| 8 | Et (1b) | Ph (3c) | rt, 20 h |  | 4f | 64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, I.; Imakuni, A.; Baba, A.; Shibata, I. Catalytic Annulation of Epoxides with Heterocumulenes by the Indium-Tin System. Molecules 2018, 23, 782. https://doi.org/10.3390/molecules23040782
Suzuki I, Imakuni A, Baba A, Shibata I. Catalytic Annulation of Epoxides with Heterocumulenes by the Indium-Tin System. Molecules. 2018; 23(4):782. https://doi.org/10.3390/molecules23040782
Chicago/Turabian StyleSuzuki, Itaru, Akira Imakuni, Akio Baba, and Ikuya Shibata. 2018. "Catalytic Annulation of Epoxides with Heterocumulenes by the Indium-Tin System" Molecules 23, no. 4: 782. https://doi.org/10.3390/molecules23040782
APA StyleSuzuki, I., Imakuni, A., Baba, A., & Shibata, I. (2018). Catalytic Annulation of Epoxides with Heterocumulenes by the Indium-Tin System. Molecules, 23(4), 782. https://doi.org/10.3390/molecules23040782