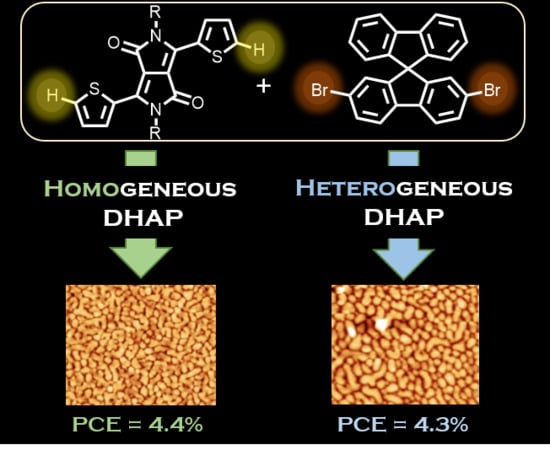
Direct (Hetero)Arylation Polymerization of a Spirobifluorene and a Dithienyl-Diketopyrrolopyrrole Derivative: New Donor Polymers for Organic Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Measurements and Characterization
3.2. Synthetic Procedures
3.3. Polymerization of PDPPSBF in Homogeneous Conditions
3.4. Polymerization of PDPPSBF in Heterogeneous Conditions
3.5. Device Fabrication and Testing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Inganäs, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Po, R.; Bianchi, G.; Carbonera, C.; Pellegrino, A. “All That Glisters Is Not Gold”: An Analysis of the Synthetic Complexity of Efficient Polymer Donors for Polymer Solar Cells. Macromolecules 2015, 48, 453–461. [Google Scholar] [CrossRef]
- Kirchartz, T.; Kaienburg, P.; Baran, D. Figures of Merit Guiding Research on Organic Solar Cells. J. Phys. Chem. C 2018, 122, 5829–5843. [Google Scholar] [CrossRef]
- Min, J.; Luponosov, Y.N.; Cui, C.; Kan, B.; Chen, H.; Wan, X.; Chen, Y.; Ponomarenko, S.A.; Li, Y.; Brabec, C.J. Evaluation of Electron Donor Materials for Solution-Processed Organic Solar Cells via a Novel Figure of Merit. Adv. Energy Mater. 2017, 7, 1700465. [Google Scholar] [CrossRef]
- Po, R.; Roncali, J. Beyond efficiency: Scalability of molecular donor materials for organic photovoltaics. J. Mater. Chem. C 2016, 4, 3677–3685. [Google Scholar] [CrossRef]
- Nitti, A.; Po, R.; Bianchi, G.; Pasini, D. Direct Arylation Strategies in the Synthesis of π-Extended Monomers for Organic Polymeric Solar Cells. Molecules 2017, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, F.; Yu, J.; Zhang, S.; Cabanetos, C.; Gao, Y.; Huang, W. Eco-friendly direct (hetero)-arylation polymerization: Scope and limitation. J. Mater. Chem. C 2017, 5, 29–40. [Google Scholar] [CrossRef]
- Bura, T.; Blaskovits, J.T.; Leclerc, M. Direct (Hetero)arylation Polymerization: Trends and Perspectives. J. Am. Chem. Soc. 2016, 138, 10056–10071. [Google Scholar] [CrossRef] [PubMed]
- Grenier, F.; Goudreau, K.; Leclerc, M. Robust Direct (Hetero)arylation Polymerization in Biphasic Conditions. J. Am. Chem. Soc. 2017, 139, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.G.; Leclerc, M. Direct (Hetero)Arylation: A New Tool for Polymer Chemists. Acc. Chem. Res. 2013, 46, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, J.-R.; Grenier, F.; Blaskovits, J.T.; Beaupré, S.; Leclerc, M. Direct (Hetero)arylation Polymerization: Simplicity for Conjugated Polymer Synthesis. Chem. Rev. 2016, 116, 14225–14274. [Google Scholar] [CrossRef] [PubMed]
- Bura, T.; Beaupre, S.; Legare, M.-A.; Quinn, J.; Rochette, E.; Blaskovits, J.T.; Fontaine, F.-G.; Pron, A.; Li, Y.; Leclerc, M. Direct heteroarylation polymerization: Guidelines for defect-free conjugated polymers. Chem. Sci. 2017, 8, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.; Wang, H.; Zhang, S. Novel Conjugated Polymers Prepared by Direct (Hetero) arylation: An Eco-Friendly Tool for Organic Electronics. Molecules 2018, 23, 408. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular Materials for Organic Photovoltaics: Small is Beautiful. Adv. Mater. 2014, 26, 3821–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrunie, A.; Jiang, Y.; Baert, F.; Leliege, A.; Roncali, J.; Cabanetos, C.; Blanchard, P. Small molecular push-pull donors for organic photovoltaics: Effect of the heterocyclic [small pi]-spacer. RSC Adv. 2015, 5, 102550–102554. [Google Scholar] [CrossRef]
- Jiang, Y.; Cabanetos, C.; Allain, M.; Liu, P.; Roncali, J. Manipulation of the band gap and efficiency of a minimalist push-pull molecular donor for organic solar cells. J. Mater. Chem. C 2015, 3, 5145–5151. [Google Scholar] [CrossRef] [Green Version]
- Grolleau, J.; Gohier, F.; Allain, M.; Legoupy, S.; Cabanetos, C.; Frère, P. Rapid and green synthesis of complementary D-A small molecules for organic photovoltaics. Org. Electron. 2017, 42, 322–328. [Google Scholar] [CrossRef]
- McAfee, S.M.; Dayneko, S.V.; Josse, P.; Blanchard, P.; Cabanetos, C.; Welch, G.C. Simply Complex: The Efficient Synthesis of an Intricate Molecular Acceptor for High-Performance Air-Processed and Air-Tested Fullerene-Free Organic Solar Cells. Chem. Mater. 2017, 29, 1309–1314. [Google Scholar] [CrossRef]
- Josse, P.; Dalinot, C.; Jiang, Y.; Dabos-Seignon, S.; Roncali, J.; Blanchard, P.; Cabanetos, C. Phthalimide end-capped thienoisoindigo and diketopyrrolopyrrole as non-fullerene molecular acceptors for organic solar cells. J. Mater. Chem. A 2016, 4, 250–256. [Google Scholar] [CrossRef]
- Josse, P.; Labrunie, A.; Dalinot, C.; McAfee, S.M.; Dabos-Seignon, S.; Roncali, J.; Welch, G.C.; Blanchard, P.; Cabanetos, C. Effect of side chains on the electronic and photovoltaic properties of diketopyrrolopyrrole-based molecular acceptors. Org. Electron. 2016, 37, 479–484. [Google Scholar] [CrossRef]
- Labrunie, A.; Josse, P.; Dabos-Seignon, S.; Blanchard, P.; Cabanetos, C. Pentaerythritol based push-pull tetramers for organic photovoltaics. Sustain. Energy Fuels 2017, 1, 1921–1927. [Google Scholar] [CrossRef]
- Graham, K.R.; Cabanetos, C.; Jahnke, J.P.; Idso, M.N.; El Labban, A.; Ngongang Ndjawa, G.O.; Heumueller, T.; Vandewal, K.; Salleo, A.; Chmelka, B.F.; et al. Importance of the Donor:Fullerene Intermolecular Arrangement for High-Efficiency Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 9608–9618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendsbee, A.D.; Sun, J.-P.; Rutledge, L.R.; Hill, I.G.; Welch, G.C. Electron deficient diketopyrrolopyrrole dyes for organic electronics: Synthesis by direct arylation, optoelectronic characterization, and charge carrier mobility. J. Mater. Chem. A 2014, 2, 4198–4207. [Google Scholar] [CrossRef]
- Guo, Q.; Dong, J.; Wan, D.; Wu, D.; You, J. Modular Establishment of a Diketopyrrolopyrrole-Based Polymer Library via Pd-Catalyzed Direct C–H (Hetero)arylation: A Highly Efficient Approach to Discover Low-Bandgap Polymers. Macromol. Rapid Commun. 2013, 34, 522–527. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Tian, H.; Zhang, J.; Yan, D.; Geng, Y.; Wang, F. High Mobility Ambipolar Diketopyrrolopyrrole-Based Conjugated Polymer Synthesized Via Direct Arylation Polycondensation. Adv. Mater. 2015, 27, 6753–6759. [Google Scholar] [CrossRef] [PubMed]
- Areephong, J.; Hendsbee, A.D.; Welch, G.C. Facile synthesis of unsymmetrical and [small pi]-extended furan-diketopyrrolopyrrole derivatives through C-H direct (hetero)arylation using a heterogeneous catalyst system. New J. Chem. 2015, 39, 6714–6717. [Google Scholar] [CrossRef]
- Lemay, M.; Pandarus, V.; Simard, M.; Marion, O.; Tremblay, L.; Béland, F. SiliaCat® S-Pd and SiliaCat DPP-Pd: Highly Reactive and Reusable Heterogeneous Silica-Based Palladium Catalysts. Top. Catal. 2010, 53, 1059–1062. [Google Scholar] [CrossRef]
- Hendriks, K.H.; Li, W.; Heintges, G.H.L.; van Pruissen, G.W.P.; Wienk, M.M.; Janssen, R.A.J. Homocoupling Defects in Diketopyrrolopyrrole-Based Copolymers and Their Effect on Photovoltaic Performance. J. Am. Chem. Soc. 2014, 136, 11128–11133. [Google Scholar] [CrossRef] [PubMed]
- Bohra, H.; Wang, M. Direct C-H arylation: A “Greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 2017, 5, 11550–11571. [Google Scholar] [CrossRef]
- Lombeck, F.; Komber, H.; Gorelsky, S.I.; Sommer, M. Identifying Homocouplings as Critical Side Reactions in Direct Arylation Polycondensation. ACS Macro Lett. 2014, 3, 819–823. [Google Scholar] [CrossRef]
- Breukelaar, W.B.; McAfee, S.M.; Welch, G.C. Exploiting direct heteroarylation polymerization homocoupling defects for the synthesis of a molecular dimer. New J. Chem. 2018, 42, 1617–1621. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yamamoto, S.-I.; Koizumi, T. Effects of molecular weight on the optical and electrochemical properties of EDOT-based π-conjugated polymers. Sci. Rep. 2017, 7, 1078. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.L.; Shin, Y.; Liu, J.; Lin, X. Structure and Optical Bandgap Relationship of π-Conjugated Systems. PLoS ONE 2014, 9, e86370. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J. Molecular Engineering of the Band Gap of π-Conjugated Systems: Facing Technological Applications. Macromol. Rapid Commun. 2007, 28, 1761–1775. [Google Scholar] [CrossRef]
- Lombeck, F.; Komber, H.; Fazzi, D.; Nava, D.; Kuhlmann, J.; Stegerer, D.; Strassel, K.; Brandt, J.; Mendaza, A.D.D.Z.; Müller, C.; et al. On the Effect of Prevalent Carbazole Homocoupling Defects on the Photovoltaic Performance of PCDTBT:PC71BM Solar Cells. Adv. Energy Mater. 2016, 6, 1601232. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Hörmann, U.; Lorch, C.; Hinderhofer, A.; Gerlach, A.; Gruber, M.; Kraus, J.; Sykora, B.; Grob, S.; Linderl, T.; Wilke, A.; et al. Voc from a Morphology Point of View: The Influence of Molecular Orientation on the Open Circuit Voltage of Organic Planar Heterojunction Solar Cells. J. Phys. Chem. C 2014, 118, 26462–26470. [Google Scholar] [CrossRef]
- Perez, M.D.; Borek, C.; Forrest, S.R.; Thompson, M.E. Molecular and Morphological Influences on the Open Circuit Voltages of Organic Photovoltaic Devices. J. Am. Chem. Soc. 2009, 131, 9281–9286. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Lundstrom, M.S.; Alam, M.A. Can morphology tailoring improve the open circuit voltage of organic solar cells? Appl. Phys. Lett. 2012, 100, 013307. [Google Scholar] [CrossRef]
Sample Availability: Not Available |




| Polymer PDPPSBF | Catalyst (mol %) | Ligand (mol %) | Yield (%) | Mn (g·mol−1) | Đm |
|---|---|---|---|---|---|
| P1 | Pd(OAc)2 (5) | PCy3•HBF4 (10) | 88 | 11,800 | 2.9 |
| P2 | Herrmann–Beller (5) | P(o-OMePh)3 (10) | 49 | 2800 | 1.5 |
| P3 | SiliaCat® DPP-Pd (5) | --- | --- | --- | --- |
| P4 | SiliaCat® DPP-Pd (10) | --- | 88 | 10,900 | 2.3 |
| Polymer PDPPSBF | λmax (nm) in CHCl3 | λmax (nm) Film | λonset (nm) Film | Ered_onset (V) | Eox_onset (V) | ELUMO (eV) | EHOMO (eV) |
|---|---|---|---|---|---|---|---|
| P1 | 648; 601; 416; 370 | 649; 608; 422; 376 | 720 | −1.45 | 0.33 | −3.35 | −5.13 |
| P2 | 638; 599; 412; 366 | 652; 601; 412; 377 | 700 | −1.36 | 0.63 | −3.44 | −5.43 |
| P4 | 649; 605; 418; 367 | 650; 608; 422; 375 | 813 | −1.44 | 0.28 | −3.36 | −5.08 |
| Polymer PDPPSBF | Voc (V) | Jsc (mA·cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| P1 | 0.87 ± 0.03 (0.88) | 11.47 ± 0.64 (11.84) | 41 ± 1 (43) | 4.10 ± 0.28 (4.47) |
| P2 | 0.80 ± 0.02 (0.81) | 11.04 ± 0.77 (11.14) | 32 ± 3 (36) | 2.87 ± 0.33 (3.27) |
| P4 | 0.87 ± 0.03 (0.89) | 11.51 ± 0.65 (11.42) | 41 ± 2 (43) | 4.13 ± 0.35 (4.31) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Josse, P.; Dayneko, S.; Zhang, Y.; Dabos-Seignon, S.; Zhang, S.; Blanchard, P.; Welch, G.C.; Cabanetos, C. Direct (Hetero)Arylation Polymerization of a Spirobifluorene and a Dithienyl-Diketopyrrolopyrrole Derivative: New Donor Polymers for Organic Solar Cells. Molecules 2018, 23, 962. https://doi.org/10.3390/molecules23040962
Josse P, Dayneko S, Zhang Y, Dabos-Seignon S, Zhang S, Blanchard P, Welch GC, Cabanetos C. Direct (Hetero)Arylation Polymerization of a Spirobifluorene and a Dithienyl-Diketopyrrolopyrrole Derivative: New Donor Polymers for Organic Solar Cells. Molecules. 2018; 23(4):962. https://doi.org/10.3390/molecules23040962
Chicago/Turabian StyleJosse, Pierre, Sergey Dayneko, Yangqian Zhang, Sylvie Dabos-Seignon, Shiming Zhang, Philippe Blanchard, Gregory C. Welch, and Clément Cabanetos. 2018. "Direct (Hetero)Arylation Polymerization of a Spirobifluorene and a Dithienyl-Diketopyrrolopyrrole Derivative: New Donor Polymers for Organic Solar Cells" Molecules 23, no. 4: 962. https://doi.org/10.3390/molecules23040962
APA StyleJosse, P., Dayneko, S., Zhang, Y., Dabos-Seignon, S., Zhang, S., Blanchard, P., Welch, G. C., & Cabanetos, C. (2018). Direct (Hetero)Arylation Polymerization of a Spirobifluorene and a Dithienyl-Diketopyrrolopyrrole Derivative: New Donor Polymers for Organic Solar Cells. Molecules, 23(4), 962. https://doi.org/10.3390/molecules23040962