Exploration of the Fluorescent Properties and the Modulated Activities against Sirtuin Fluorogenic Assays of Chromenone-Derived Natural Products
Abstract
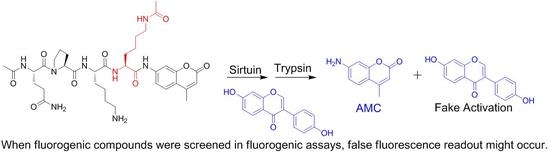
:1. Introduction
2. Results and Discussion
2.1. The Fluorescence Properties of Chromenone Derived Natural Products
2.2. The SIRT1/2 Activities of Chromenone Derived Natural Products
2.3. The Inhibitory Mode of Osthole against SIRT2
3. Materials and Methods
3.1. The Chromenone Derived Natural Products
3.2. Comparison of Fluorescent Properties of Chromenone Derived Natural Products
3.3. The Purification of SIRT1 and SIRT2 Recombinant Proteins
3.4. Sirtuin Inhibition Assay
3.5. The Inhibition Mode Study of Osthole against SIRT2
3.6. The Docking Study of Osthole Binding to SIRT2
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Acknowledgments
Conflicts of Interest
References
- Teles, Y.C.F.; Souza, M.S.R.; Souza, M.F.V. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health: A Review of Emerging Biologic Pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-Lopez, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Cui, H.; Jin, J.; Lai, F.; Wen, H.; Zhang, X.; Ruda, G.F.; Chen, X.; Yin, D. Development of 3-alkyl-6-methoxy-7-hydroxy-chromones (AMHCs) from natural isoflavones, a new class of fluorescent scaffolds for biological imaging. Chem. Commun. 2015, 51, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Shvadchak, V.V.; Fauerbach, J.A.; Jares-Erijman, E.A.; Jovin, T.M. Highly Solvatochromic 7-Aryl-3-hydroxychromones. J. Phys. Chem. Lett. 2012, 3, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; He, Y.; Wen, H.; Lai, F.; Yin, D.; Cui, H. A Suzuki-Miyaura method for labelling proliferating cells containing incorporated BrdU. Analyst 2018, 143, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, S.S.; Brem, J.; Rydzik, A.M.; Salimraj, R.; Cain, R.; Verma, A.; Owens, R.J.; Fishwick, C.W.; Spencer, J.; Schofield, C.J. Assay platform for clinically relevant metallo-beta-lactamases. J. Med. Chem. 2013, 56, 6945–6953. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Cui, H.; Chen, L. Multi-targeted histone deacetylase inhibitors in cancer therapy. Curr. Med. Chem. 2012, 19, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kamal, Z.; Ai, T.; Xu, Y.; More, S.S.; Wilson, D.J.; Chen, L. Discovery of potent and selective sirtuin 2 (SIRT2) inhibitors using a fragment-based approach. J. Med. Chem. 2014, 57, 8340–8357. [Google Scholar] [CrossRef] [PubMed]
- Woronoff, G.; El Harrak, A.; Mayot, E.; Schicke, O.; Miller, O.J.; Soumillion, P.; Griffiths, A.D.; Ryckelynck, M. New generation of amino coumarin methyl sulfonate-based fluorogenic substrates for amidase assays in droplet-based microfluidic applications. Anal. Chem. 2011, 83, 2852–2857. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yu, P.; Wang, Y.; Mao, L. Real-time ratiometric fluorescent assay for alkaline phosphatase activity with stimulus responsive infinite coordination polymer nanoparticles. Anal. Chem. 2015, 87, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, D.; Karpenko, I.A.; Barthes, N.P.; Michel, B.Y.; Klymchenko, A.S.; Benhida, R.; Demchenko, A.P.; Mely, Y.; Burger, A. Rational design of a solvatochromic fluorescent uracil analogue with a dual-band ratiometric response based on 3-hydroxychromone. Chemistry 2014, 20, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, K.; Grun, A.; Stamm, A.; Schmitt, Y.; Gerhards, M.; Diller, R. ESIPT and photodissociation of 3-hydroxychromone in solution: Photoinduced processes studied by static and time-resolved UV/Vis, fluorescence, and IR spectroscopy. J. Phys. Chem. A 2013, 117, 11233–11245. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Chen, W.Y. Sorting out functions of sirtuins in cancer. Oncogene 2014, 33, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Lin, H. Sirtuins in epigenetic regulation. Chem. Rev. 2015, 115, 2350–2375. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. Calorie restriction increases life span: A molecular mechanism. Nutr. Rev. 2006, 64 Pt 1, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, A.Z.; Guarente, L. SIRT1 in neurodevelopment and brain senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; Martinez-Redondo, P.; Marazuela-Duque, A.; Vazquez, B.N.; Dooley, S.J.; Voigt, P.; Beck, D.B.; Kane-Goldsmith, N.; Tong, Q.; Rabanal, R.M.; et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 2013, 27, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Finnin, M.S.; Donigian, J.R.; Pavletich, N.P. Structure of the histone deacetylase SIRT2. Nat. Struct. Biol. 2001, 8, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Galleano, I.; Schiedel, M.; Jung, M.; Madsen, A.S.; Olsen, C.A. A Continuous, Fluorogenic Sirtuin 2 Deacylase Assay: Substrate Screening and Inhibitor Evaluation. J. Med. Chem. 2016, 59, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Schutkowski, M.; Fischer, F.; Roessler, C.; Steegborn, C. New assays and approaches for discovery and design of Sirtuin modulators. Expert Opin. Drug Discov. 2014, 9, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; Hallows, W.C.; Denu, J.M. A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal. Biochem. 2009, 394, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ludewig, R.; Scriba, G.K. 9-Fluorenylmethoxycarbonyl-labeled peptides as substrates in a capillary electrophoresis-based assay for sirtuin enzymes. Anal. Biochem. 2009, 387, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, P.A.; Richardson, P.L.; Guo, J.; Barrett, L.W.; Xu, N.; Gunasekera, A.; Glaser, K.B. Fluorescence assay of SIRT protein deacetylases using an acetylated peptide substrate and a secondary trypsin reaction. Anal. Biochem. 2004, 332, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bitterman, K.J.; Anderson, R.M.; Cohen, H.Y.; Latorre-Esteves, M.; Sinclair, D.A. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 2002, 277, 45099–45107. [Google Scholar] [CrossRef] [PubMed]
- Disch, J.S.; Evindar, G.; Chiu, C.H.; Blum, C.A.; Dai, H.; Jin, L.; Schuman, E.; Lind, K.E.; Belyanskaya, S.L.; Deng, J.; et al. Discovery of thieno[3,2-d]pyrimidine-6-carboxamides as potent inhibitors of SIRT1, SIRT2, and SIRT3. J. Med. Chem. 2013, 56, 3666–3679. [Google Scholar] [CrossRef] [PubMed]
- Friden-Saxin, M.; Seifert, T.; Landergren, M.R.; Suuronen, T.; Lahtela-Kakkonen, M.; Jarho, E.M.; Luthman, K. Synthesis and evaluation of substituted chroman-4-one and chromone derivatives as sirtuin 2-selective inhibitors. J. Med. Chem. 2012, 55, 7104–7113. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Khan, M.N.; Sawada, H.; Imai, E.; Itoh, Y.; Yamatsuta, K.; Tokuda, N.; Takeuchi, J.; Seko, T.; Nakagawa, H.; et al. Design, synthesis, and biological activity of a novel series of human sirtuin-2-selective inhibitors. J. Med. Chem. 2012, 55, 5760–5773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, H.; Yu, X.; Peng, T.; Wang, G.; Wen, X.; Sun, Y.; Liu, S.; Zhang, S.; Hu, L.; et al. Synthesis and Evaluation of Novel Benzofuran Derivatives as Selective SIRT2 Inhibitors. Molecules 2017, 22, 1348. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Rasbach, K.A.; Schnellmann, R.G. Isoflavones promote mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 2008, 325, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Kustigian, L.; Carney, D.; Case, A.; Considine, T.; Hubbard, B.P.; Perni, R.B.; Riera, T.V.; Szczepankiewicz, B.; Vlasuk, G.P.; et al. SIRT1 activation by small molecules: Kinetic and biophysical evidence for direct interaction of enzyme and activator. J. Biol. Chem. 2010, 285, 32695–32703. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.M.; Alcain, F.J. Sirtuin activators and inhibitors. Biofactors 2012, 38, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Martin-Montalvo, A.; Mercken, E.M.; Palacios, H.H.; Ward, T.M.; Abulwerdi, G.; Minor, R.K.; Vlasuk, G.P.; Ellis, J.L.; Sinclair, D.A.; et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014, 6, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Hirasaka, K.; Maeda, T.; Ikeda, C.; Haruna, M.; Kohno, S.; Abe, T.; Ochi, A.; Mukai, R.; Oarada, M.; Eshima-Kondo, S.; et al. Isoflavones derived from soy beans prevent MuRF1-mediated muscle atrophy in C2C12 myotubes through SIRT1 activation. J. Nutr. Sci. Vitaminol. 2013, 59, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; E, S.Y.; Lamming, D.W.; et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Ruda, G.F.; Carrero-Lerida, J.; Ruiz-Perez, L.M.; Gilbert, I.H.; Gonzalez-Pacanowska, D. Exploring new inhibitors of Plasmodium falciparum purine nucleoside phosphorylase. Eur. J. Med. Chem. 2010, 45, 5140–5149. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Cui, Q.; Meng, H.; Lai, F.; Wang, S.; Zhang, X.; Chen, X.; Cui, H.; Yin, D. A high-resolution method to assess cell multinucleation with cytoplasm-localized fluorescent probes. Analyst 2016, 141, 4010–4013. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Shen, Z.; Wen, H.; Chen, J.; Zhang, X.; Lin, P.; Yin, D.; Cui, H.; Chen, X. A Morphological identification cell cytotoxicity assay using cytoplasm-localized fluorescent probe (CLFP) to distinguish living and dead cells. Biochem. Biophys. Res. Commun. 2017, 482, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y.; Shen, S.; Wen, H.; Cui, H. A Mild Aqueous Sonogashira Reaction as a Fluorescent Labeling Strategy for 5-Bromide-2′-Deoxyuridine. Molecules 2018, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the authors. |




| Compound | Structure | Fluorescence | Activity (10 μM) | |||
|---|---|---|---|---|---|---|
| Ex | Em | φ | SIRT1 | SIRT2 | ||
| 1 |  | 304 | 400 | 0.16 | 11.60 | 0.87 |
| 2 |  | - | - | - | 0.76 | 0.72 |
| 3 |  | - | - | - | 0.95 | 0.83 |
| 4 |  | - | - | - | 0.82 | 0.47 |
| 5 |  | - | - | - | 1.21 | 0.75 |
| 6 |  | - | - | - | 3.22 | 0.80 |
| 7 |  | - | - | - | 1.10 | 0.86 |
| 8 |  | - | - | - | 0.89 | 0.80 |
| 9 |  | - | - | - | 0.65 | 0.81 |
| 10 |  | - | - | - | 0.82 | 0.76 |
| 11 |  | - | - | - | 0.90 | 0.79 |
| 12 |  | - | - | - | 0.92 | 0.79 |
| 13 |  | 340 | 490 | 0.05 | 0.92 | 0.80 |
| 14 |  | 338 | 488 | 0.08 | 0.80 | 0.95 |
| 15 |  | 340 | 490 | 0.03 | 0.87 | 0.83 |
| 16 |  | - | - | - | 1.00 | 0.72 |
| 17 |  | 348 | 478 | 0.04 | 0.79 | 0.66 |
| 18 |  | - | - | - | 0.81 | 0.77 |
| 19 |  | - | - | - | 1.00 | 0.93 |
| 20 |  | - | - | - | 0.96 | 0.93 |
| 21 |  | - | - | - | 0.93 | 0.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Xue, N.; Wu, F.; He, Y.; Zhang, G.; Hu, Z.; Cui, H. Exploration of the Fluorescent Properties and the Modulated Activities against Sirtuin Fluorogenic Assays of Chromenone-Derived Natural Products. Molecules 2018, 23, 1063. https://doi.org/10.3390/molecules23051063
Wen H, Xue N, Wu F, He Y, Zhang G, Hu Z, Cui H. Exploration of the Fluorescent Properties and the Modulated Activities against Sirtuin Fluorogenic Assays of Chromenone-Derived Natural Products. Molecules. 2018; 23(5):1063. https://doi.org/10.3390/molecules23051063
Chicago/Turabian StyleWen, Hui, Nina Xue, Feng Wu, Yujun He, Gang Zhang, Zebin Hu, and Huaqing Cui. 2018. "Exploration of the Fluorescent Properties and the Modulated Activities against Sirtuin Fluorogenic Assays of Chromenone-Derived Natural Products" Molecules 23, no. 5: 1063. https://doi.org/10.3390/molecules23051063
APA StyleWen, H., Xue, N., Wu, F., He, Y., Zhang, G., Hu, Z., & Cui, H. (2018). Exploration of the Fluorescent Properties and the Modulated Activities against Sirtuin Fluorogenic Assays of Chromenone-Derived Natural Products. Molecules, 23(5), 1063. https://doi.org/10.3390/molecules23051063