3.5. General Procedure for the Preparation of Fluoroethoxychalcones
An aqueous solution of KOH (1.2 eq.) was added at room temperature to a solution of the appropriate acetophenone (1.0 eq.), and benzaldehyde (1.2 eq.) in ethanol (10 mL). The reaction mixture was then stirred for 30 min. After completion of reaction, the reaction was judged by TLC. The reaction mixture was filtered through a Buckner funnel under vacuum. The solid was washed several times with a 1:1 ethanol-water mixture. The solid was finally dried under vacuum. The crude product was further purified by column chromatography or recrystallization.
(E)-3-(4-Methylphenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (
1a) [
43]. Yellow crystalline solid, Yield: 87%, m.p. 148–150 °C (lit. 148–151 °C);
1H-NMR (CDCl
3)
δ (ppm): 2.40 (s, 3H), 4.44 (q, 2H,
3J = 8.0 Hz), 7.03 (d, 2H,
3J = 8.7 Hz), 7.23 (d, 2H,
3J = 8.8 Hz) 7.48 (d, 1H,
3J = 15.2 Hz), 7.55 (d, 2H,
3J = 8.4 Hz), 7.80 (d, 1H,
3J = 15.6 Hz), 8.06 (d, 2H,
3J = 8.8 Hz).
(E)-3-(4-(Dimethylamino)phenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1b). Yellow solid, Yield: 77%, m.p. 130–135 °C; 1H-NMR (CDCl3) δ (ppm): 3.05 (s, 6H), 4.43 (q, 2H, 3J = 8.0 Hz), 6.70 (d, 2H, 3J = 8.4 Hz), 7.02 (d, 2H, 3J = 9.1 Hz), 7.33 (d, 1H, 3J = 15.2 Hz), 7.55 (d, 2H, 3J = 8.4 Hz), 7.79 (d, 1H, 3J = 15.2 Hz), 8.04 (d, 2H, 3J = 9.1 Hz); 13C-NMR (CDCl3) δ (ppm): 40.26, 65.65 (q, 2JC-F = 36.2 Hz), 111.92, 114.53, 116.43, 122.71, 130.54, 130.75, 133.63, 145.75, 152.14, 160.36, 188.95; 19F-NMR (CDCl3) δ (ppm): −73.7(t, 3F,3J = 8.7 Hz); IR (KBr) ν: 3070, 2909, 1650, 1585, 1528, 1371, 1343, 1287, 1231, 1162, 1069, 1027, 969, 808, 676; HRMS (ESI) calcd. for C19H18F3NO2: [M + H]+ 350.1368 and [M + Na]+ 372.1187; Found: 350.1366 and 372.1163.
(E)-3-(4-Chlorophenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1c). Pale yellow solid, Yield: 90%, m.p. 139–140 °C; 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 8.0 Hz), 7.04 (d, 2H, 3J = 8.8 Hz), 7.40 (d, 2H, 3J = 8.4 Hz), 7.49 (d, 1H, 3J = 15.4 Hz), 7.58 (d, 2H, 3J = 8.4 Hz), 7.76 (d, 1H, 3J = 15.4 Hz), 8.05 (d, 2H, 3J = 8.8 Hz); 13C-NMR (CDCl3) δ (ppm): 65.70 (q, 2JC-F = 36.7 Hz), 114.79, 122.16, 129.41, 129.72, 131.06, 132.70, 133.54, 136.57, 143.23, 160.95, 188.50; 19F-NMR (CDCl3) δ (ppm): −73.67 (t, 3F, 3J = 8.6 Hz); IR (KBr) ν: 3066, 2947, 2815, 1663, 1609, 1510, 1490, 1406, 1343, 1293, 1162, 1029, 972, 811, 692, 657; HRMS (ESI) calc. for C17H12ClF3O2: [M + H]+ 341.0056; Found: 341.056.
(E)-3-(4-Bromophenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1d). Pale yellow solid, Yield: 77%, m.p. 145–146 °C; 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 8.0 Hz), 7.04 (d, 2H, 3J = 9.1 Hz), 7.49–7.57 (m, 5H), 7.74 (m, 1H), 8.05 (d, 2H, 3J = 9.1 Hz); 13C-NMR (CDCl3) δ (ppm): 65.70 (q, 2JC-F = 36.6 Hz), 114.79, 122.25, 124.93, 129.92, 131.06, 132.37, 132.68, 133.96, 143.30, 160.95, 188.49; 19F-NMR (CDCl3) δ (ppm): −73.67 (t, 3F, 3J = 8.7 Hz); IR (KBr) ν: 2946, 2812, 1668, 1609, 1511, 1487, 1349, 1305, 1293, 1254, 1180, 1161, 1079, 1028, 972, 808, 687; HRMS (ESI) calc. for C17H12BrF3O2: [M + H]+ 385.0051; Found: 385.0037.
(E)-3-(3-Nitrophenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (
1e) [
43]. Yellow solid, Yield: 94%, m.p. 169–171 °C (lit. 168–171 °C);
1H-NMR (CDCl
3)
δ (ppm): 4.46 (q, 2H,
3J = 7.9 Hz), 7.07 (d, 2H,
3J = 9.1 Hz), 7.62 (d, 1H,
3J = 15.9 Hz), 7.84 (d, 1H,
3J = 15.9 Hz) 7.92 (d, 1H,
3J = 7.6 Hz), 8.09 (d, 2H,
3J = 8.5 Hz), 8.26 (d, 1H,
3J = 8.5 Hz), 8.52 (s, 1H).
(E)-3-(2,5-Dimethoxyphenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (
1f) [
43]. Yellow solid, Yield: 78%, m.p. 98–99 °C (lit. 97–99 °C);
1H-NMR (CDCl
3)
δ (ppm): 3.82 (s, 3H), 3.87 (s, 3H), 4.44 (q, 2H,
3J = 7.9 Hz), 6.87–6.89 (m, 1H), 6.93–6.96 (m, 1H), 7.03 (d, 2H,
3J = 8.8 Hz), 7.16–7.17 (m, 1H), 7.57 (d, 1H,
3J = 15.9 Hz), 8.04–8.09 (m, 3H).
(E)-3-(Furan-2-yl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (
1g) [
43]. Light yellow crystalline solid, Yield: 61%, m.p. 94–96 °C (lit. 94.5–96.5 °C);
1H-NMR (CDCl
3)
δ (ppm): 4.43 (q, 2H,
3J = 8.0 Hz), 6.52 (m, 1H), 6.72 (d, 1H,
3J = 3.5 Hz), 7.03 (d, 2H,
3J = 8.7 Hz), 7.45 (d, 1H,
3J = 15.3 Hz), 7.52 (s, 1H), 7.59 (d, 1H,
3J = 15.3 Hz), 8.07 (d, 2H,
3J = 8.7 Hz).
(E)-3-(5-Bromothiophen-2-yl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1h). Pale yellow solid, Yield: 93%, yield, m.p. 93–94 °C; 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 8.0 Hz), 7.03 (d, 2H, 3J = 8.7 Hz), 7.06 (d, 1H, 3J = 4.2 Hz), 7.10 (d, 1H, 3J = 4.2 Hz), 7.21 (d, 1H, 3J = 15.6 Hz), 7.81 (d, 1H, 3J = 15.6 Hz), 8.02 (d, 2H, 3J = 8.7 Hz); 13C-NMR (CDCl3) δ (ppm): 65.71 (q, 2JC-F = 36.0 Hz), 114.80, 116.48, 120.69, 130.96, 131.51, 132.49, 132.60, 136.20, 142.12, 160.96, 187.84; 19F-NMR (CDCl3) δ (ppm): −73.68 (t, 3F, 3J = 7.6 Hz); IR (KBr) ν: 3068, 2829, 1648, 1605, 1510, 1422, 1353, 1294, 1249, 1168, 1159, 1069, 1028, 972, 826, 796, 675 cm−1; HRMS (ESI) calcd. for C15H10BrF3O2S: [M + H]+ 390.9615; Found: 390.9608.
(E)-3-(2-Bromophenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1i). Pale yellow solid, Yield: 81%, m.p. 107–108 °C; 1H-NMR (CDCl3) δ (ppm): 4.43 (q, 2H, 3J = 7.9 Hz), 7.02 (d, 2H, 3J = 8.5 Hz), 7.24 (m, 1H), 7.34 (m, 1H), 7.39 (d, 1H, 3J = 15.7 Hz), 7.63 (d, 1H, 3J = 7.3 Hz), 7.71 (d, 1H,3J = 7.6 Hz), 8.04 (d, 2H, 3J = 8.5 Hz), 8.12 (d, 1H, 3J = 15.7 Hz); 13C-NMR (CDCl3) δ (ppm): 65.69 (q, 2JC-F = 36.1 Hz), 114.77, 123.20 (q, 1JC-F = 277.60 Hz), 124.88, 125.99, 127.85, 128.02, 131.21, 131.45, 132.55, 133.72, 135.25, 143.11, 160.94, 188.78; 19F-NMR (CDCl3) δ (ppm): −73.67 (t, 3F, 3J = 7.7 Hz); IR (KBr) ν: 3072, 2952, 2821, 1660, 1607, 1511, 1463, 1435, 1344, 1299, 1220, 1184, 1076, 1027, 970, 865, 830, 757, 739, 688, 657 cm−1; HRMS (ESI) calcd. for C17H12BrF3O2: [M + H]+ 385.0051; Found: 385.0051.
(E)-3-(3-Bromophenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1j). Pale yellow solid, Yield: 74%, m.p. 104–106 °C; 1H-NMR (CDCl3) δ (ppm): 4.45 (q, 2H, 3J = 7.9 Hz), 7.04 (d, 2H, 3J = 8.9 Hz), 7.28–7.32 (m, 1H), 7.50–7.55 (m, 3H), 7.73 (m, 1H), 7.80 (s, 1H), 8.07 (d, 2H,3J = 8.9 Hz); 13C-NMR (CDCl3) δ (ppm): 65.70 (q, 2JC-F = 36.4 Hz), 114.80, 122.95, 123.24, 127.43, 130.63, 130.90, 131.11, 132.57, 133.38, 137.17, 142.87, 161.01, 188.32; 19F-NMR (CDCl3) δ (ppm): −73.63 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 3064, 2948, 2894, 2361, 1663, 1611, 1511, 1418, 1341, 1255, 1220, 1182, 1127, 1078, 1030, 971, 830, 780, 669, 661 cm−1; HRMS (ESI) calcd. for C17H12BrF3O2: [M + H]+ 385.0051; Found: 385.0019.
(E)-3-(4-Methoxyphenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1k). Yellow solid, Yield: 72%, m.p. 100–102 °C; 1H-NMR (CDCl3) δ (ppm): 3.86 (s, 3H), 4.44 (q, 2H, 3J = 7.9 Hz), 6.94 (d, 2H, 3J = 8.8 Hz), 7.03 (d, 2H, 3J = 8.8 Hz),7.41 (d, 1H, 3J = 15.9 Hz), 7.61 (d, 2H, 3J = 8.8 Hz), 7.79 (d, 1H, 3J = 15.9 Hz), 8.05 (d, 2H, 3J = 8.8 Hz); 13C-NMR (CDCl3) δ (ppm): 55.56, 65.69 (q, 2JC-F = 35.9 Hz), 114.57, 114.67, 119.41, 127.77, 130.36, 130.93, 133.15, 144.61, 160.68, 161.82, 188.85; 19F-NMR (CDCl3) δ (ppm): −73.69 (t, 3F, 3J = 7.7 Hz); IR (KBr) ν: 3042, 3073, 2957, 2839, 1657, 1603, 1572, 1510, 1423, 1344, 1295, 1249, 1168, 1033, 976, 813, 680 cm−1; HRMS(ESI) calcd. for C18H15F3O3: [M + H]+ 337.1051 and [M + Na]+ 359.0871; Found: 37.1051 and 359.0864.
(E)-3-(3-Methoxyphenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1l). Pale yellow solid, Yield: 91%, m.p. 78–80 °C; 1H-NMR (CDCl3) δ (ppm): 3.86 (s, 3H), 4.44 (q, 2H, 3J = 7.9 Hz), 6.96–6.98 (m, 1H), 7.04 (d, 2H, 3J = 8.7 Hz), 7.15–7.16 (m, 1H), 7.23 (s, 1H), 7.34 (t, 1H, 3J = 8.2 Hz), 7.54 (d, 1H, 3J = 15.7 Hz), 7.77 (d, 1H, 3J = 15.7 Hz), 8.06 (d, 2H, 3J = 8.7 Hz); 13C-NMR (CDCl3) δ (ppm): 55.51, 65.63 (q, 2JC-F = 39.0 Hz), 113.63, 114.73, 116.37, 121.21, 122.06, 130.12, 131.07, 132.82, 136.41, 144.68, 160.09, 160.84, 188.82; 19F-NMR (CDCl3) δ (ppm): −73.66 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 3079, 3004, 2941, 2833, 1650, 1606, 1510, 1426, 1314, 1264, 1217, 1158, 1026, 978, 818, 677 cm−1; HRMS (ESI) Calc. for C18H15F3O3: [M + H]+ 337.1051 and [M + Na]+ 359.0871; Found: 337.1048 and 359.0865.
(E)-3-(3,4-Dimethoxyphenyl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1m). Yellow solid, Yield: 70%, m.p. 116–118 °C; 1H-NMR (CDCl3) δ (ppm): 3.94 (s, 3H), 3.96 (s, 3H), 4.44 (q, 2H, 3J = 7.9 Hz), 6.90 (d, 1H, 3J = 8.9 Hz), 7.04 (d, 2H, 3J = 8.7 Hz), 7.16 (d, 1H, 3J = 1.9 Hz), 7.24 (dd, 1H, 3J = 8.3 Hz, 4J = 1.9 Hz) 7.38 (d, 1H, 3J = 15.7 Hz), 7.77 (d, 1H, 3J = 15.7 Hz), 8.05 (d, 2H, 3J = 8.7 Hz); 13C-NMR (CDCl3) δ (ppm): 56.12, 56.15, 65.69 (q, 2JC-F = 36.0 Hz), 110.23, 111.26, 119.69, 123.28, 128.00, 130.96, 133.10, 144.95, 149.39, 151.58, 160.70, 188.89; 19F-NMR (CDCl3) δ (ppm): −73.67 (t, 3F, 3J= 7.6 Hz); IR (KBr) ν: 3079, 3003, 2941, 2833, 1651, 1606, 1590, 1510, 1425, 1361, 1236, 1217, 1180, 1141, 978, 817, 791, 677 cm−1; HRMS (ESI) calcd. for C19H17F3O4: [M + H]+ 367.1157 and [M + Na]+ 389.0977; Found: 367.1157 and 389.0987.
(E)-3-(1H-Indol-2-yl)-1-(4-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (1n). Yellow solid, Yield: 40%, m.p. 194–195 °C; 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 8.0 Hz), 7.05 (d, 2H, 3J = 8.0 Hz), 7.31–7.33 (m, 2H), 7.45–7.47 (m, 1H), 7.57–7.62 (m, 2H), 8.03 (dd, 1H, 3J = 6.7 Hz, 4J = 3.0 Hz), 8.09–8.13 (m, 3H), 8.61 (s, 1H); 13C-NMR (CDCl3) δ (ppm): 65.68 (q, 2JC-F = 35.9), 112.14, 114.64, 117.50, 120.84, 121.92, 123.72, 125.48, 130.46, 130.80, 133.55, 137.38, 138.83, 160.47, 189.26; 19F-NMR (CDCl3) δ (ppm): −73.68 (t, 3F, 3J = 8.7 Hz); IR (KBr) ν: 3219, 2929, 1647, 1604, 1587, 1554, 1365, 1246, 1170, 1041, 815, 735, 677 cm−1; HRMS (ESI) calcd. for C19H14F3NO2: [M + H]+ 346.1055; Found: 346.1051.
(E)-3-(4-Methylphenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2a). Yellow solid, Yield: 80%, m.p. 107–108 °C; 1H-NMR (CDCl3) δ (ppm): 2.42 (s, 3H), 4.45 (q, 2H, 3J = 8.1 Hz), 7.20 (dd, 1H, 3J = 8.2 Hz, 4J = 2.5 Hz), 7.24–7.27 (m, 2H), 7.46–7.50 (m, 2H), 7.56–7.58 (m, 3H), 7.72 (d, 1H, 3J = 8.0 Hz), 7.83 (d, 1H, 3J = 15.6 Hz); 13C-NMR (CDCl3) δ (ppm): 21.70, 65.99 (q, 2JC-F = 36.1 Hz), 113.82, 120.10, 120.79, 122.84, 128.72, 129.90, 130.10, 132.12, 140.13, 141.50, 145.62, 157.76, 189.88; 19F-NMR (CDCl3) δ (ppm): −73.7 (t, 3F, 3J = 7.8 Hz); IR (KBr) ν: 3083, 3049, 2360, 1658, 1595, 1580, 1454, 1325, 1305, 1247, 1161, 1076, 1041, 967, 812, 800, 741, 674 cm−1; HRMS (ESI) Calc. for C18H15F3O2: [M + H]+ 321.1124; Found: 321.1093.
(E)-3-(4-(Dimethylamino)phenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2b). Yellow solid, Yield: 71%, m.p. 134–136 °C; 1H-NMR (CDCl3) δ (ppm): 3.05 (s, 6H), 4.44 (q, 2H, 3J = 8.1 Hz), 6.69 (d, 2H, 3J = 9.2 Hz), 7.16 (dd, 1H, 3J= 8.3 Hz, 4J = 2.5 Hz), 7.30 (d, 1H, 3J = 15.6 Hz), 7.44 (t, 1H, 3J = 7.9 Hz), 7.54–7.57 (m, 3H), 7.69 (d, 1H, 3J = 7.9 Hz), 7.81 (d, 1H, 3J = 15.6 Hz); 13C-NMR (CDCl3) δ (ppm): 40.27, 66.00 (q, 2JC-F = 35.8 Hz), 111.96, 113.69, 116.56, 119.62, 122.61, 122.67, 129.93, 130.71, 140.92, 146.54, 152.30, 157.69, 189.86; 19F-NMR (CDCl3) δ (ppm): −73.71 (t, 3F, 3J = 7.6Hz); HRMS (ESI calc. for C19H18F3NO2: [M + H]+ 350.1368; Found: 350.1362.
(E)-3-(4-Chlorophenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2c). Yellow solid, Yield: 73%, m.p. 117–118 °C; 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 8.1 Hz), 7.20 (dd, 1H, 3J = 8.8 Hz, 4J = 2.7 Hz) 7.40–7.42 (m, 2H), 7.46–7.50 (m, 2H), 7.58–7.60 (m, 3H), 7.70 (d, 1H, 3J = 7.9 Hz), 7.78 (d, 1H, 3J = 15.7 Hz); 13C-NMR (CDCl3) δ (ppm): 65.99 (q, 2JC-F = 35.8 Hz), 113.90, 120.31, 122.17, 122.86, 129.45, 129.81, 130.19, 133.34, 136.80, 139.80, 143.97, 157.82, 189.50; 19F-NMR (CDCl3) δ (ppm): −73.76 (t, 3F, 3J = 8.7 Hz); IR (KBr) ν: 3104, 3081, 2945, 2360, 1664, 1610, 1581, 1490, 1451, 1295, 1273, 1250, 1170, 1152, 1045, 973, 914, 868, 821, 789, 690 cm−1; HRMS (ESI) calc. for C17H12ClF3O2: [M + H]+ 341.0556 and [M + Na]+ 363.0376; Found: 341.0555 and 363.0375.
(E)-3-(4-Bromophenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2d). Pale yellow solid, Yield: 75%, m.p. 125–126 °C; 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 8.0 Hz), 7.20 (dd, 1H, 3J = 8.4 Hz, 4J = 2.7 Hz), 7.45–7.58 (m, 7H), 7.70 (d, 1H, 3J = 8.4 Hz), 7.76 (d, 1H, 3J = 15.6 Hz); 13C-NMR (CDCl3) δ (ppm): 66.64 (q, 2JC-F = 36.5 Hz), 113.96, 120.32, 122.39, 122.88, 125.19, 130.01, 130.20, 132.41, 133.79, 139.90, 144.03, 157.84, 189.75; 19F-NMR (CDCl3) δ (ppm): −73.7 (t, 3F, 3J = 7.7 Hz); IR (KBr) ν: 3076, 2945, 2360, 1667, 1599, 1486, 1450, 1401, 1314, 1295, 1170, 1152, 1080, 1045, 973, 867, 816, 788, 688 cm−1; HRMS (ESI) calc. for C17H12BrF3O2: [M + H]+ 385.0051 and [M + Na]+ 406.9871; Found: 385.0054 and 406.9879.
(E)-3-(3-Nitrophenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2e). Brown solid, Yield: 73%, m.p. 78–80 °C; 1H-NMR (CDCl3) δ (ppm): 4.45 (q, 2H, 3J = 8.0 Hz), 7.23 (dd, 1H, 3J = 8.5 Hz, 4J = 3.0 Hz), 7.50 (t, 1H, 3J = 8.1 Hz), 7.59–7.65 (m, 3H), 7.74 (d, 1H, 3J = 7.8 Hz), 7.85 (m, 1H), 7.93 (d, 1H, 3J = 7.2 Hz), 8.27 (dd, 3J = 8.0 Hz, 4J = 2.7 Hz), 8.51–8.8.52 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 66.01 (q, 2JC-F = 35.2 Hz), 113.95, 120.67, 122.58, 122.98, 124.36, 130.25, 130.34, 134.51, 136.62, 139.33, 142.28, 148.89, 157.91, 188.96; 19F-NMR (CDCl3) δ (ppm): −73.39 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 3074, 2869, 2360, 1662, 1591, 528, 1444, 1357, 1309,1272, 1248, 1173, 1158, 1083, 1068, 974, 911, 857, 795, 780, 740, 675 cm−1; HRMS (ESI) calc. for C17H12F3NO4: [M + H]+ 352.0797; Found: 352.0789.
(E)-3-(2,5-Dimethoxyphenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2f). Yellow solid, Yield: 77%, m.p. 72–74 °C; 1H-NMR (CDCl3) δ (ppm): 3.83 (s, 3H), 3.88 (s, 3H), 4.44 (q, 2H, 3J = 8.1 Hz), 6.89 (m, 1H), 6.94–6.97 (m,1H), 7.16–7.18 (m, 1H), 7.20 (d, 1H, 4J = 2.7 Hz), 7.46 (t, 1H, 3J = 8.0 Hz), 7.53–7.57 (m, 2H), 7.69 (d, 1H, 3J = 8.4 Hz), 8.09 (d, 1H, 3J = 16 Hz); 13C-NMR (CDCl3) δ (ppm): 56.01, 56.25, 66.05 (q, 2JC-F = 35.90 Hz), 112.64, 113.90, 113.99, 117.60, 120.10, 122.01, 122.96, 124.49, 130.06, 140.25, 140.86, 153.56, 153.70, 157.77, 190.40; 19F-NMR (CDCl3) δ (ppm): −73.7 (t, 3F, 3J = 7.7 Hz); IR (KBr) ν: 2948, 2838, 2357, 1659, 1587, 1494, 1442, 1323, 1248, 1172, 1123, 1047, 985, 974, 848, 791, 693 cm−1; HRMS (ESI) calc. for C19H17F3O4: [M + H]+ 367.1157; Found: 367.1158.
(E)-3-(Furan-2-yl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2g). Brown viscous liquid, Yield: 65%, 1H-NMR (CDCl3) δ (ppm): 4.39 (q, 2H, 3J = 7.90 Hz), 6.44–6.45 (m, 1H), 6.64 (d, 1H, 3J = 3.5 Hz), 6.9 (d, 1H, 3J = 8.4 Hz), 7.09–7.13 (m,1H), 7.21–7.22 (m, 1H), 7.36–7.46 (m, 3H), 7.64 (d, 1H, 3J = 8.0 Hz); 13C-NMR (CDCl3) δ (ppm): 65.58 (q, 2H, 3J = 36.2 Hz,) 112.94, 113.61, 116.87, 118.98, 120.29, 122.83, 130.14, 131.26, 139.89, 145.29, 157.76, 189.14; 19F-NMR (CDCl3) δ (ppm): −73.71 (t, 3J = 8.7 Hz); IR (KBr) ν: 3126, 1688, 1586, 1486, 1442, 1401, 1250, 1169, 1069, 1016, 973, 858, 789, 734, 680 cm−1; HRMS (ESI) calc. for C15H11F3O3: [M + H]+ 297.0739; Found: 297.0730.
(E)-3-(5-Bromothiophen-2-yl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2h). Yellow solid, Yield: 85%, m.p. 103–105 °C; 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 8.1 Hz), 7.07 (m, 1H), 7.12 (m, 1H), 7.17–7.21 (m, 2H), 7.45–7.48 (m, 1H), 7.54–7.55 (m, 1H), 7.66 (d, 1H, 3J = 7.6 Hz), 7.83 (d, 1H, 3J = 15.7 Hz); 13C-NMR (CDCl3) δ (ppm): 65.94 (q, 2JC-F = 36.1 Hz), 113.65, 116.90, 120.35, 120.59, 121.94, 122.74, 130.19, 131.55, 132.84, 136.85, 139.65, 141.89, 157.78, 188.82; 19F-NMR (CDCl3) δ (ppm): −73.75 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 3073, 2941, 1658, 1579, 1488, 1423, 1346, 1264, 1247, 1162, 1070, 1057, 970, 917, 854, 814, 783, 716, 697 cm−1; HRMS (ESI) calc. for C15H10BrF3O2S: [M + H]+ 390.9615; Found: 390.961.
(E)-3-(4-Methoxyphenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2k). Yellow solid, Yield: 73%, m.p. 79–82 °C; 1H-NMR (CDCl3) δ (ppm): 3.86 (s, 3H) 4.44 (q, 2H, 3J = 8.0 Hz), 6.94 (d, 2H, 3J = 8.3 Hz), 7.18 (dd, 1H, 3J = 8.6 Hz, 4J = 2.6 Hz),7.38 (d, 1H, 3J = 15.6 Hz),7.46 (t, 1H, 3J = 8.2 Hz), 7.56–7.57 (m, 1H), 7.61 (d, 2H, 3J = 8.4 Hz), 7.70 (d, 1H, 3J = 8.0 Hz), 7.80 (d, 1H, 3J = 15.6 Hz); 13C-NMR (CDCl3) δ (ppm): 55.57, 65.95 (q, 2JC-F = 35.40), 113.75, 114.59, 119.43, 119.98, 122.77, 127.57, 130.07, 130.51, 140.27, 145.38, 157.73, 161.98, 189.81; 19F-NMR (CDCl3) δ (ppm): −73.7 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 3075, 3014, 2941, 2843, 1655, 1587, 1573, 1442, 1342, 1287, 1245, 1187, 1156, 1056, 1027, 972, 921, 872, 824, 798, 788, 669 cm−1; HRMS (ESI) calc. for C18H15F3O3: [M + H]+ 337.1052; Found: 337.1048.
(E)-3-(3,4-Dimethoxyphenyl)-1-(3-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (2m). Yellow solid, Yield: 64%, 1H-NMR (CDCl3) δ (ppm): 3.94 (s, 3H), 3.96 (s, 3H), 4.44 (q, 2H, 3J = 8.1 Hz), 6.91 (d, 1H, 3J = 8.0 Hz), 7.17–7.18 (m,1H), 7.19–7.24 (dd, 1H),7.36 (d, 1H, 3J = 15.6 Hz), 7.47 (t, 1H, 3J = 8.0 Hz), 7.57 (s, 1H), 7.70 (d, 1H, 3J = 8.0 Hz), 7.78 (d, 1H, 3J = 15.6 Hz); 13C-NMR (CDCl3) δ (ppm): 55.94, 56.11, 65.94 (q, 2JC-F = 36.1 Hz), 110.14, 111.20, 113.85, 119.68, 119.89, 122.78, 123.53, 127.80, 130.06, 140.23, 145.73, 149.37, 151.71, 157.72, 189.85; 19F-NMR (CDCl3) δ (ppm): −73.75 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 3128, 2840, 1656, 1576, 1517, 1421, 1256, 1165, 1080, 1026, 977, 919, 785, 724, 679 cm−1; HRMS (ESI) Calc. for C19H17F3O4: [M + H]+ 367.1157 and [M + Na]+ 389.0977; Found: 367.1154 and 389.0979.
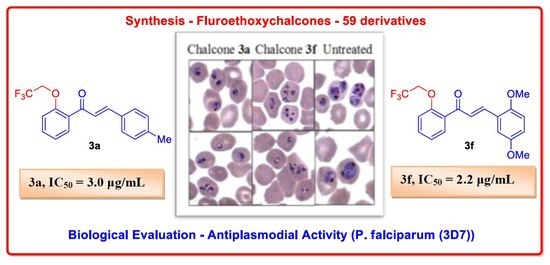
(E)-3-(4-Methylphenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3a). Pale yellow solid, Yield: 82%, m.p. 80–82 °C; 1H-NMR (CDCl3) δ (ppm): 2.38 (s, 3H), 4.44 (q, 2H, 3J = 8.0 Hz), 6.96 (d, 1H, 3J = 8.0 Hz), 7.15–7.17 (m, 1H), 7.20 (d, 2H, 3J = 8.0 Hz), 7.38 (m, 1H), 7.49 (d, 2H, 3J = 7.6 Hz), 7.64 (m, 1H), 7.70 (d, 1H, 3J = 7.8 Hz), 7.78 (d, 1H, 3J = 7.8 Hz); 13C-NMR (CDCl3) δ (ppm): 21.66, 66.53 (q, 2JC-F = 35.8 Hz), 113.21, 123.05, 125.55, 128.65, 129.78, 130.36, 131.19, 132.27, 133.02, 141.11, 144.30, 155.60, 192.08; 19F-NMR (CDCl3) δ (ppm): −73.51 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 3115.70, 1648.15, 1604.14, 1484.69, 1449.97, 1403.88, 1330.59, 1265.53, 1163.65, 119.59, 978, 813.90, 761.55, 682.04 cm−1; HRMS (ESI) calc. for C18H15F3O2: [M + H]+ 321.1102; Found: 321.1095.
(E)-3-(4-(Dimethylamino)phenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3b). Yellow solid, Yield: 72%, m.p. 64–66 °C; 1H-NMR (CDCl3) δ (ppm): 3.05 (s, 6H), 4.44 (q, 2H, 3J = 8.1 Hz), 6.69–6.71 (m, 2H), 7.14–7.17 (m, 1H), 7.28–7.32 (m,1H), 7.44 (t, 1H), 7.54–7.57 (m, 3H), 7.68–7.70 (m, 1H), 7.79–7.83 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 40.27, 65.65 (q, 2JC-F = 36.20 Hz), 111.93, 114.53, 116.43, 121.87, 122.72, 124.63, 130.54, 130.75, 133.63, 145.75, 152.14, 160.36, 188.96; 19F-NMR (CDCl3) δ (ppm): −74.19 (t, 3F, 3J = 7.6 Hz); IR (KBr) ν: 2821, 2359, 1646, 1599, 1520, 1484, 1447, 1339, 1266, 1233, 1162, 1064, 1025, 970, 861, 815, 767, 753, 676 cm−1; HRMS (ESI) calc. for C19H18F3NO2: [M + H]+ 350.1368; Found: 350.1366.
(E)-3-(4-Chlorophenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3c). Yellow solid, Yield: 73%, m.p. 61–63 °C; 1H-NMR (CDCl3) δ (ppm): 4.45 (q, 2H, 3J = 8.0 Hz), 6.95 (d, 1H, 3J = 8.4 Hz), 7.17 (t, 1H, 3J = 7.7 Hz), 7.36–7.40 (m, 3H), 7.49–7.53 (m, 3H), 7.62 (m, 1H), 7.73 (dd, 1H, 3J = 7.6 Hz, 4J = 1.9 Hz); 13C-NMR (CDCl3) δ (ppm): 66.37 (q, 2JC-F = 36.0 Hz), 112.94, 123.10, 126.91, 129.32, 129.73, 129.91, 131.40, 133.42, 133.58, 136.41, 142.31, 155.69, 191.49; 19F-NMR (CDCl3) δ (ppm): −73.70 (t, 3F, 3J = 7.6 Hz); IR (KBr) ν: 2884, 2361, 1651, 1602, 1588, 1485, 1447, 1330, 1260, 1227, 1196, 1069, 1024, 979, 863, 821, 769, 693 cm−1; HRMS (ESI) calc. for C17H12ClF3O2: [M + H]+ 341.0556 and [M + Na]+ 363.0376;Found: 341.0545 and 363.0363.
(E)-3-(4-Bromophenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3d). Yellow solid, Yield: 73%, m.p. 68–71 °C; 1H-NMR (CDCl3) δ (ppm): 4.45 (q, 2H, 3J = 8.0 Hz), 6.95 (d, 1H, 3J = 8.0 Hz), 7.17 (t, 1H, 3J = 7.4 Hz), 7.43 (d, 1H, 3J = 15.9 Hz), 7.45 (d, 2H, 3J = 8.0 Hz), 7.53 (d, 2H, 3J = 8.5 Hz), 7.61 (d, 1H, 3J = 15.9 Hz), 7.73 (d, 1H, 3J = 7.9 Hz); 13C-NMR (CDCl3) δ (ppm): 66.34 (q, 2JC-F = 35.1 Hz), 112.89, 123.09, 124.62, 124.78, 126.98, 129.92, 131.40, 132.27, 133.44, 133.99, 142.33, 155.68, 191.46; 19F-NMR (CDCl3) δ (ppm): −73.54 (t, 3F, 3J = 7.6 Hz); IR (KBr) ν: 2855, 2361, 1652, 1603, 1586, 1486, 1450, 1329, 1276, 1198, 1071, 1022, 819, 764, 692 cm−1; HRMS (ESI) calc. for C17H12BrF3O2: [M + H]+ 385.0051; Found: 385.0042.
(E)-3-(3-Nitrophenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3e). Brown solid, Yield: 75%, m.p. 170–172 °C; 1H-NMR (CDCl3) δ (ppm): 4.48 (q, 2H, 3J = 7.9 Hz), 6.96 (d, 1H, 3J = 8.4 Hz), 7.17–7.21 (m, 1H), 7.53–7.61 (m, 3H), 7.69–7.72 (m, 1H), 7.78 (dd, 1H, 3J = 7.7 Hz, 4J = 1.9 Hz), 7.89 (d, 1H, 3J = 8.1 Hz), 8.24 (d, 1H, 3J = 8.8 Hz), 8.46 (s, 1H); 13C-NMR (CDCl3) δ (ppm): 66.21 (q, 2JC-F = 35.7 Hz), 112.66, 122.72, 123.14, 124.64, 129.04, 129.33, 130.06, 131.59, 133.92, 134.12, 136.91, 140.25, 148.87, 155.83, 190.86; 19F-NMR (CDCl3) δ (ppm): −73.67 (t, 3F, 3J = 8.7 Hz); IR (KBr) ν: 3113, 3093, 2954, 2359, 1651, 1604, 1527, 1487, 1448, 1331, 1271, 1230, 1061, 1021, 979, 865, 769, 747, 670 cm−1; HRMS (ESI) calc. for C17H12F3NO4: [M + H]+ 352.0796 and [M + Na]+ 374.0616; Found: 352.0798 and 374.0616.
(E)-3-(2,5-Dimethoxyphenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3f). Yellow solid, Yield: 62%, 77–79 °C; 1H-NMR (CDCl3) δ (ppm): 3.79 (s, 3H), 3.82 (s, 3H), 4.43 (q, 2H, 3J = 8.1 Hz), 6.83–6.86 (m, 1H), 6.91–6.96 (m, 2H), 7.11–7.17 (m, 2H), 7.39–7.43 (m, 1H), 7.46–7.50 (m, 1H), 7.69 (dd, 1H, 3J = 7.7 Hz, 4J = 2.1 Hz), 8.02 (d, 1H, 3J = 16.0 Hz); 13C-NMR (CDCl3) δ (ppm): 55.84, 56.24, 66.64 (q, 2JC-F = 35.8 Hz), 112.38, 112.69, 113.39, 118.18, 123.08, 124.51, 126.82, 130.61, 131.20, 132.91, 139.20, 153.43, 153.69, 155.63, 192.34; 19F-NMR (CDCl3) δ (ppm): −73.45 (t, 3F, 3J = 8.8 Hz); IR (KBr) ν: 2942, 2836, 2358, 1657, 1601, 1587, 1497, 1338, 1220, 1164, 1043, 978, 863, 820, 751, 680 cm−1; HRMS (ESI) calc. for C19H17F3O4: [M + H]+ 367.1157; Found: 367.1132.
(E)-3-(Furan-2-yl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3g). Brown solid, Yield: 59%, m.p. 72–74 °C, 1H-NMR (CDCl3) δ (ppm): 4.44 (q, 2H, 3J = 7.9 Hz), 6.48–6.50 (m, 1H), 6.69 (d, 1H, 3J = 3.5 Hz), 6.95 (d, 1H, 3J = 8.4 Hz), 7.14–7.18 (m, 1H), 7.27–7.31 (m, 1H), 7.41–7.51 (m, 3H), 7.69 (d, 1H, 3J = 8 Hz); 13C-NMR (CDCl3) δ (ppm): 66.63 (q, 2JC-F = 36.0 Hz), 112.68, 113.41, 115.88, 123.05, 124.17, 130.15, 130.27, 131.11, 133.08, 145.17, 151.79, 155.73, 191.56; 19F-NMR (CDCl3) δ (ppm): −73.54 (t, 3F, 3J = 8.7 Hz); IR (KBr) ν: 3040, 2901, 2360, 2342, 1645, 1603, 1587, 1487, 1449, 1330, 1295, 1206, 1016, 969, 868, 771, 746, 675 cm−1; HRMS (ESI) calc. for C15H11F3O3: [M + H]+ 297.0738; Found: 297.0735.
(E)-3-(5-Bromothiophen-2-yl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3h). Yellow solid, Yield: 87%, m.p. 94–95 °C; 1H-NMR (CDCl3) δ (ppm): 4.45 (q, 2H, 3J = 8.0 Hz), 6.94 (d, 1H, 3J = 8.4 Hz), 7.03–7.07 (m, 2H), 7.14–7.18 (m, 2H), 7.48–7.53 (m, 1H), 7.67–7.73 (m, 2H); 13C-NMR (CDCl3) δ (ppm): 66.64 (q, 2JC-F = 36.3 Hz), 113.00, 116.60, 123.08, 123.23 (q, 1JC-F = 279.6 Hz), 125.52, 129.83, 131.39, 132.20, 133.41, 135.25, 142.20, 142.60, 155.73, 190.75; 19F-NMR (CDCl3) δ (ppm): −73.3 (t, 3F, 3J = 7.5 Hz); IR (KBr) ν: 3096, 2361, 1645, 1580, 1597, 1568, 1427, 1347, 1296, 1270, 1170, 1159, 1023, 979, 966, 867, 773, 675 cm−1; HRMS (ESI) calc. for C18H15F3O2: [M + H]+ 321.1102; Found: 321.1093.
(E)-3-(2-Bromophenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3i). Pale yellow solid, Yield: 74%, m.p. 76–77 °C; 1H-NMR (CDCl3) δ (ppm): 4.45 (q, 2H, 3J = 8.0 Hz), 6.96 (d, 1H, 3J = 8.4 Hz), 7.18 (t, 1H, 3J = 7.8 Hz), 7.20–7.23 (m, 1H), 7.32–7.37 (m, 2H), 7.49–7.54 (m, 1H), 7.61 (d, 1H, 3J = 7.4 Hz), 7.70–7.74 (m, 2H), 8.02 (d, 1H, 3J = 15.7 Hz); 13C-NMR (CDCl3) δ (ppm): 66.47 (q, 2JC-F = 35.9 Hz), 113.03, 121.86, 123.12, 126.17, 127.83, 127.88, 128.99, 129.89, 131.37, 131.41, 133.36, 133.59, 142.33, 155.68, 191.73; 19F-NMR (CDCl3) δ (ppm): −73.48 (t, 3F, 3J = 6.5 Hz); IR (KBr) ν: 3060, 2902, 2342, 1646, 1604, 1486, 1450, 1329, 1278, 1201, 1149, 1120, 1023, 970, 864, 753, 693 cm−1; HRMS (ESI) calc. for C17H12BrF3O2: [M + H]+ 385.0051 and [M + Na]+ 406.9871; Found: 385.0042 and 406.9854.
(E)-3-(3-Bromophenyl)-1-(2-(2,2,2-trifluoroethoxy)phenyl)prop-2-en-1-one (3j). Yellow solid, Yield: 74%, m.p. 97–98 °C; 1H-NMR (CDCl3) δ (ppm): 4.47 (q, 2H, 3J = 7.9 Hz), 6.96 (d, 1H, 3J = 8.4 Hz), 7.17–7.21 (m, 1H), 7.27–7.30 (m, 2H), 7.43–7.47 (m, 1H), 7.52 (d, 2H, 3J = 8.4 Hz), 7.58–7.62 (m, 1H), 7.73–7.76 (m, 2H); 13C-NMR (CDCl3) δ (ppm): 66.36 (q, 2JC-F = 36.1 Hz), 112.90, 123.08, 127.14, 127.67, 129.76, 130.51, 131.20, 131.40, 133.23, 133.52, 137.20, 141.90, 155.72, 191.33; 19F-NMR (CDCl3) δ (ppm): −73.49 (t, 3F, 3J = 7.7 Hz); IR (KBr) ν: 3039, 2870, 2361, 2343, 1647, 1605, 1588, 1451, 1325, 1289, 1266, 1119, 1056, 1024, 969, 865, 767, 667 cm−1; HRMS (ESI) calc. for C17H12BrF3O2: [M + H]+ 385.0051 and [M + Na]+ 406.9871; Found: 385.0057 and 406.9874.
(E)-1-(4-(2-Fluoroethoxy)phenyl)-3-(4-methylphenyl)-prop-2-en-1-one (
4a) [
44]. Yellow solid, Yield: 78%, m.p. 110.1–110.7 °C;
1H-NMR (CDCl
3)
δ (ppm): 2.39 (s, 3H), 4.30 (dt, 2H,
3JH-H = 3.8 Hz,
3JH-F = 28.1 Hz), 4.80 (dt, 2H,
3JH-H = 4.1 Hz,
2JH-F = 47.2 Hz), 7.00–7.02 (m, 2H), 7.22–7.24 (m, 2H), 7.48–7.56 (m, 3H), 7.77–7.81(m,1H), 8.04(d, 2H, J = 8.4 Hz).
(E)-1-(4-(2-Fluoroethoxy)phenyl)-3-(3-nitrophenyl)prop-2-en-1-one (
4e) [
44]. Pale yellow solid, Yield: 66%, m.p. 116–118 °C (lit. 115–117 °C);
1H-NMR (CDCl
3)
δ (ppm): 4.32 (dt, 2H,
3JH-H = 4.0 Hz,
3JH-F = 27.1 Hz,), 4.81 (dt, 2H,
3JH-H = 3.9 Hz,
2JH-F = 47.1 Hz,), 7.04 (m, 2H), 7.60–7.69 (m, 2H), 7.81–7.85 (m, 1H), 7.91 (d, 1H,
3J = 7.7 Hz), 8.08 (d, 2H,
3J = 8.7 Hz), 8.25 (d, 1H,
3J = 8.0 Hz), 8.52 (s, 1H).
(E)-3-(2,5-Dimethoxyphenyl)-1-(4-(2-fluoroethoxy)phenyl)prop-2-en-1-one (4f). Yellow solid, Yield: 80%, m.p. 76–77 °C (lit. 75–77 °C); 1H-NMR (CDCl3) δ (ppm): 3.82 (s, 3H), 3.87 (s, 3H), 4.30 (dt, 2H, 3JH-H = 4.9 Hz, 3JH-F = 25.6 Hz), 4.80 (dt, 2H, 3JH-H = 4.7 Hz, 2JH-F = 46.4 Hz), 6.87–6.89 (m, 1H), 6.92–6.95 (m, 1H), 7.01 (d, 2H, 3J = 8.8 Hz), 7.16–7.17 (m, 1H), 7.57–7.61 (m, 1H), 8.02–8.09 (m, 3H); 13C-NMR (CDCl3) δ (ppm): 55.99, 56.26, 67.31 (d, 2JC-F = 20.7 Hz), 81.82 (d, 1JC-F = 170.9 Hz), 112.57, 113.92, 114.45, 117.13, 123.03, 124.78, 131.01, 131.43, 131.97, 139.67, 153.42, 153.62, 162.15, 189.46; 19F-NMR (CDCl3) δ (ppm): −223.52 to −223.92 (m, 1F); IR (KBr) ν: 2946, 2838, 1656, 1607, 1598, 1572, 1496, 1453, 1427, 1341, 1318, 1281, 1249, 1185, 1116, 1073, 1052, 974, 950 cm−1; HRMS (ESI) calcd. for C19H19FO4: [M + H]+ 331.1346 and [M + Na]+ 353.1165; Found: 331.1359 and 353.1171.
(E)-1-(4-(2-Fluoroethoxy)phenyl)-3-(furan-2-yl)prop-2-en-1-one (
4g) [
44]. Brown solid, Yield: 67%, m.p. 75–76 °C;
1H-NMR (CDCl
3)
δ (ppm): 4.30 (dt, 2H,
3JH-H = 4.1 Hz,
3JH-F = 26.9 Hz), 4.79 (dt, 2H,
3JH-H = 3.5 Hz,
2JH-F = 48.0 Hz), 6.51 (dd, 1H,
3J = 3.1 Hz,
3J = 1.7 Hz), 6.7 (d, 1H,
3J = 3.9 Hz), 6.99–7.02 (m, 2H), 7.44–7.60 (m, 3H), 8.04–8.06 (m, 2H).
(E)-3-(2-Bromophenyl)-1-(4-(2-fluoroethoxy)phenyl)prop-2-en-1-one (4i). Pale yellow solid, Yield: 76%, m.p. 115–116 °C; 1H-NMR (CDCl3) δ (ppm): 4.31 (dt, 2H, 3JH-H = 3.8 Hz, 3JH-F = 28.3 Hz), 4.80 (dt, 2H, 3JH-H = 4.0 Hz, 2JH- F = 47.5 Hz), 7.02 (d, 2H, 3J = 8.8 Hz), 7.32–7.45 (m, 3H), 7.64 (d, 1H, 3J = 8.1 Hz), 7.72–7.74 (m, 1H), 8.03–8.06 (m, 2H), 8.10–8.14 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 67.35 (d, 2JC-F = 20.6 Hz), 81.77 (d, 1JC-F = 172.1 Hz), 114.57, 122.78, 125.03, 125.95, 127.82, 128.00, 131.17, 131.33, 133.68, 135.37, 142.69, 162.42; 19F-NMR (CDCl3) δ (ppm): −223.50 to −223.90 (m, 1F); IR (KBr) ν: 3696, 3311, 2360, 1657, 1599, 1575, 1509, 1464, 1368, 1332, 1316, 1273, 1222, 1187, 1092, 1017, 918, 886, 829, 757, 740, 671, 647, 579, 509 cm−1; HRMS (ESI) calcd. for C17H14BrFO2: [M + H]+ 349.0239; Found: 349.0233.
(E)-3-(4-Bromophenyl)-1-(3-(2-fluoroethoxy)phenyl)prop-2-en-1-one (5d). Yellow solid, Yield: 69%, m.p. 122–124 °C; 1H-NMR (CDCl3) δ (ppm): 4.30 (dt, 2H, 3JH-H = 4.0 Hz, 3JH-F = 27.9 Hz), 4.8 (dt, 2H, 3JH-H = 4.1 Hz, 2JH-F = 47.4 Hz), 7.17–7.19 (m,1H), 7.43 (t, 1H, 3J = 8.0 Hz), 7.48–7.57 (m, 6H), 7.62–7.64 (m, 1H), 7.72–7.76 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 67.45 (d, 2JC-F = 20.60 Hz), 81.90 (d, 1JC-F = 171.3 Hz), 113.65, 120.19, 121.75, 122.53, 125.03, 127.64, 129.97, 132.37, 133.86, 139.55, 143.67, 158.90, 189.88; 19F-NMR (CDCl3) δ (ppm): −223.49 to −223.89 (m, 1F); IR (KBr) ν: 2968, 1660, 1597, 1577, 1459, 1486, 1443, 1313, 1181, 1113, 1072, 1049, 1007, 975, 945, 893, 876, 834, 819, 793, 770, 691, 678, 612, 491 cm−1; HRMS (ESI) calcd. for C17H14BrFO2 : [M + H]+ 349.0239 and [M + Na]+ 371.0059; Found: 349.0233 and 371.0059.
(E)-3-(2,5-Dimethoxyphenyl)-1-(3-(2-fluoroethoxy)phenyl)prop-2-en-1-one (5f). Yellow viscous solid, Yield: 75%, 1H-NMR (CDCl3) δ (ppm): 3.82 (s, 3H), 3.87 (s, 3H), 4.30 (dt, 2H, 3JH-H = 4.2 Hz, 3JH-F = 27.9 Hz), 4.79 (dt, 2H, 3JH-H = 4.4 Hz, 2JH-F = 47.0 Hz), 6.86–6.89 (m, 1H), 6.94–6.97 (m, 1H), 7.12–7.18 (m, 2H), 7.42 (t, 1H, 3J = 8.0 Hz), 7.54–7.58 (m, 2H), 7.62–7.64 (m, 1H), 8.07–8.10 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 56.01, 56.26, 67.44 (d, 2JC-F = 20.1 Hz), 81.97 (d, 1JC-F = 171.20 Hz), 112.62, 113.62, 113.85, 117.51, 119.97, 121.86, 123.15, 124.58, 129.82, 140.04, 140.52, 153.52, 153.66, 158.81, 190.81; 19F-NMR (CDCl3) δ (ppm): −223.46 to −223.86 (m, 1F); IR (KBr) ν: 2952, 2836, 2066, 1866, 1660, 1585, 1495, 1438, 1326, 1259, 1180, 1118, 1046, 990, 946, 859, 865, 747, 710, 612, 564 cm−1; HRMS (ESI) calcd. for C19H19FO4:[M + H]+ 331.1346 and [M + Na]+ 353.1165; Found: 331.1338 and 353.1157.
(E)-1-(3-(2-Fluoroethoxy)phenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (5k). Pale yellow solid, Yield: 90%, m.p. 104–105 °C; 1H-NMR (CDCl3) δ (ppm): 3.86 (s, 3H), 4.30 (dt, 2H, 3JH-H = 4.1 Hz,3JH-F = 28.4 Hz,), 4.79 (dt, 2H, 3JH-H = 3.9 Hz, 2JH-F = 47.2 Hz), 6.93–6.95(m, 2H), 7.15–7.17 (m, 1H), 7.38–7.44(m, 2H), 7.56–7.64 (m, 4H), 7.77–7.81(m, 1H); 13C-NMR (CDCl3) δ (ppm): 55.57, 67.41 (d, 2JC-F = 20.2 Hz), 81.97 (d, 1JC-F = 170.9 Hz), 113.55, 114.57, 119.76, 119.86, 121.67, 127.69, 129.82, 130.44, 140.08, 145.04, 158.83, 161.88, 190.26; 19F-NMR (CDCl3) δ (ppm): −223.51 to −223.91 (m, 1F); IR (KBr) ν: 2972, 1657, 1594, 1575, 1511, 1486, 1418, 1326, 1309, 1286, 1254, 1182, 1172, 1112, 1074, 1046, 1030, 976, 890, 875, 830, 793, 739, 680, 536, 516 cm−1; HRMS (ESI) calcd. for C18H17FO3 :[M + H]+ 301.1240 and [M + Na]+ 323.1059; Found: 301.1233 and 323.1050.
(E)-3-(2-Bromophenyl)-1-(2-(2-fluoroethoxy)phenyl)prop-2-en-1-one (6i). Yellow solid, Yield: 76%, m.p. 86–88 °C; 1H-NMR (CDCl3) δ (ppm): 4.33 (dt, 2H, 3JH-H = 3.7 Hz, 3JH-F = 28.6 Hz), 4.76 (dt, 2H, 3JH-H = 4.0 Hz, 2JH-F = 47.5 Hz), 6.96–6.98 (m, 1H), 7.08–7.11 (m, 1H), 7.19–7.24 (m, 1H), 7.30–7.34 (m, 1H), 7.46–7.50 (m, 2H), 7.60–7.62 (m, 1H), 7.71–7.78 (m, 2H), 8.01–8.05 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 67.91 (d, 2JC-F = 19.7 Hz), 81.77 (d, 1JC-F = 171.40 Hz), 112.60, 121.75, 126.14, 127.82, 127.96, 127.98, 129.43, 131.17, 131.21, 133.43, 133.54, 135.22, 141.21, 157.17, 192.15; 19F-NMR (CDCl3) δ (ppm): −222.76 to 223.16 (m, 1F); IR (KBr) ν: 3331, 2893, 1646, 1606, 1484, 1464, 1450, 1329, 1281, 1262, 1166, 1119, 1064, 1042, 1026, 975, 922, 880, 814, 756, 670, 648, 616, 578, 530, 467, 446 cm−1; HRMS (ESI) calcd. for C17H14BrFO2: [M + H]+ 349.0239 and [M + Na]+ 371.0059; Found: 349.0243 and 371.0057.
(E)-3-(4-(2-Fluoroethoxy)phenyl)-1-phenylprop-2-en-1-one (
7i) [
44]. Yellow solid, Yield: 76%, m.p. 79–80 °C (lit. 78–80 °C);
1H-NMR (CDCl
3)
δ (ppm): 4.27 (dt, 2H,
3JH-H = 4.2 Hz,
3JH-F = 27.6 Hz), 4.79 (dt, 2H,
3JH-H = 3.8 Hz,
2JH-F = 47.6 Hz), 6.96–6.98 (m, 2H), 7.41–7.45 (m, 1H), 7.49–7.52 (m, 2H), 7.56–7.58 (m, 1H), 7.61–7.63 (m, 2H), 7.77–7.81 (m, 1H), 8.00–8.02 (m, 2H).
(E)-3-(4-(2-Fluoroethoxy)phenyl)-1-(3-nitrophenyl)prop-2-en-1-one (
7ii) [
44]. Pale yellow solid, Yield: 59%, 122–124 °C (lit. 122–124 °C);
1H-NMR (CDCl
3)
δ (ppm): 2.44 (s, 3H), 4.27 (dt, 2H,
3JH-H = 4.1 Hz,
3JH-F = 27.5 Hz), 4.78 (dt, 2H,
3JH-H = 4.2 Hz,
2JH-F = 47.4 Hz), 6.96 (d, 2H,
3J = 8.9 Hz), 7.30 (d, 2H,
3J = 8.0 Hz), 7.42–7.45 (m, 1H), 7.60–7.62 (m, 2H), 7.76–7.80 (m, 1H), 7.92–7.94 (m, 2H).
(E)-1-(2,4-Dichlorophenyl)-3-(4-(2-fluoroethoxy)phenyl)prop-2-en-1-one (7iii). Pale yellow solid, Yield: 67%, m.p. 85–87 °C; 1H-NMR (CDCl3) δ (ppm): 4.26 (dt, 2H, 3JH-H = 4.2 Hz, 3JH-F = 27.6 Hz), 4.78 (dt, 2H, 3JH-H = 4.1 Hz, 2JH-F = 47.4 Hz), 6.94–6.97 (m, 2H), 7.01 (s, 1H), 7.33–7.36 (m, 1H), 7.39–7.41 (m, 1H), 7.43–7.48 (m, 2H), 7.52–7.54 (m, 2H); 13C-NMR (CDCl3) δ (ppm): 67.27 (d, 2JC-F = 21.20 Hz), 81.76 (d, 1JC-F = 171.5 Hz), 115.22, 124.17, 127.39, 127.64, 130.29, 130.47, 130.67, 132.42, 136.84, 137.80, 146.52, 160.98, 192.82; 19F-NMR (CDCl3) δ (ppm): −223.92 to −223.52 (m, 1F); IR (KBr) ν: 3072, 2968, 1897, 1731, 1660, 1592, 1572,1516, 1456, 1427, 1374, 1337, 1296, 1253, 1267, 1208, 1180, 1048, 1025, 984, 921, 885, 863, 793, 682, 579, 519 cm−1; HRMS (ESI) calcd. for C17H13Cl2FO2: [M + H]+ 339.0355 and [M + Na]+ 361.0174; Found: 339.0342 and 361.0155.
(E)-3-(4-(2-Fluoroethoxy)phenyl)-1-(2-methoxyphenyl)prop-2-en-1-one (
7iv) [
44]. Yellow solid, Yield: 73%, m.p. 88–89 °C (lit. 87–89 °C);
1H-NMR (CDCl
3)
δ (ppm): 3.89 (s, 3H), 4.42 (dt, 2H,
3JH-H = 3.9 Hz,
3JH-F = 28.2 Hz), 4.77 (dt, 2H,
3JH-H = 4.0 Hz,
2JH-F = 47.6 Hz), 6.93 (d, 2H,
3J = 8.7 Hz), 6.98–7.05 (m, 2H), 7.22–7.25 (m, 1H), 7.46 (t, 1H,
3J = 8.0 Hz), 7.52–7.55 (m, 2H), 7.58–7.60 (m, 2H).
(E)-1-(2-Bromophenyl)-3-(4-(2-fluoroethoxy)phenyl)prop-2-en-1-one (
7vii) [
44]. Yellow solid, Yield: 71%, m.p. 107–108 °C (lit. 107–109 °C);
1H-NMR (CDCl
3)
δ (ppm): 4.27 (dt, 2H,
3JH-H = 4.1 Hz,
3JH-F = 27.7 Hz), 4.78 (dt, 2H,
3JH-H = 3.9 Hz,
2JH-F = 46.4 Hz), 6.97 (d, 2H,
3J = 7.5 Hz), 7.17 (m, 2H), 7.38–7.42 (m, 1H), 7.60–7.62 (m, 2H), 7.77–7.81 (m, 1H), 8.03–8.07 (m, 2H).
(E)-3-(3-(2-Fluoroethoxy)phenyl)-1-(4-methylphenyl)-prop-2-en-1-one (
8ii) [
44]. Yellow solid, Yield: 70%, m.p. 81–82 °C (lit. 80–82 °C);
1H-NMR (CDCl
3)
δ (ppm): 2.5 (s, 3H), 4.33 (dt, 2H,
3JH-H = 3.9 Hz,
3JH-F = 28.1 Hz), 4.85 (dt, 2H,
3JH-H = 3.7 Hz,
2JH-F = 47.7 Hz), 7.04–7.06 (m, 1H), 7.32–7.43 (m, 4H), 7.58–7.60 (m, 1H), 7.80–7.84 (m, 1H), 7.99–8.01 (m, 2H).
(E)-1-(2,4-Dichlorophenyl)-3-(3-(2-fluoroethoxy)phenyl)prop-2-en-1-one (8iii). Pale yellow solid, Yield: 80%, m.p. 101–102 °C; 1H-NMR (CDCl3) δ (ppm): 4.28 (dt, 2H, 3JH-H = 4.2 Hz, 3JH-F = 27.6 Hz), 4.76 (dt, 2H, 3JH-H = 4.4 Hz, 2JH-F = 47.9 Hz), 6.91–6.93 (m, 1H), 7.01–7.04 (m, 1H), 7.24–7.28 (m, 1H), 7.33–7.48 (m, 4H), 7.58 (d, 1H, 3J = 7.4 Hz), 7.82–7.86 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 67.87 (d, 2JC-F = 20.6 Hz), 81.71 (d, 1JC-F = 171.5 Hz), 112.59, 121.74, 123.91, 126.82, 127.30, 129.85, 130.28, 130.69, 132.40, 132.57, 136.89, 137.76, 142.10, 157.74, 193.12; 19F-NMR (CDCl3) δ (ppm): −223.56 to −223.95 (m, 1F); IR (KBr) ν: 2966, 1671, 1600, 1572, 1493, 1450, 1372, 1320, 1280, 1248, 1203, 1130, 1073, 1041, 1016, 987, 928, 887, 824, 762, 745, 575 cm−1; HRMS (ESI) calcd. for C17H13Cl2FO2: [M + H]+ 339.0355 and [M + Na]+ 361.0174; Found: 339.0350 and 361.0164.
(E)-3-(3-(2-Fluoroethoxy)phenyl)-1-(2-methoxyphenyl)prop-2-en-1-one (8iv). Pale yellow paste, Yield: 86%, 1H-NMR (CDCl3) δ (ppm): 3.89 (s, 3H), 4.28 (dt, 2H, 3JH-H = 4.3 Hz, 3JH-F = 26.9 Hz), 4.78 (dt, 2H, 3JH-H = 5.1 Hz, 2JH-F = 45.7 Hz), 6.90–6.92 (m, 1H), 6.98–7.05 (m, 3H), 7.34 (t, 3J = 7.8 Hz), 7.43–7.48 (m, 2H), 7.61 (d, 2H, 3J = 8.0 Hz), 7.94–7.98 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 55.81, 67.78 (d, 2JC-F = 20.7 Hz), 81.80 (d, 1JC-F = 171.8 Hz), 111.67, 112.44, 120.75, 121.56, 124.68, 128.02, 129.36, 129.65, 130.46, 131.54, 132.77, 138.77, 157.56, 158.17, 193.78; 19F-NMR (CDCl3) δ (ppm): −223.45 to −223.85 (m, 1F); IR (KBr) ν: 2962, 1658, 1604, 1485, 1438, 1403, 1281, 1246, 1179, 1163, 1114, 1055, 1028, 982, 886, 761, 678, 655 cm−1; HRMS (ESI) calcd. for C18H17FO3: [M + H]+ 301.1240 and [M + Na]+ 323.1059; Found: 301.1206 and 323.1029.
(E)-1-(4-Bromophenyl)-3-(3-(2-fluoroethoxy)phenyl)prop-2-en-1-one (8v). Yellow solid, Yield: 74%, m.p. 122–123 °C; 1H-NMR (CDCl3) δ (ppm): 4.33 (dt, 2H, 3JH-H = 4.0 Hz, 3JH-F = 27.9 Hz), 4.85 (dt, 2H, 3JH-H = 3.9 Hz, 2JH-F = 47.7 Hz), 7.05–7.08 (m, 1H), 7.32–7.34 (m, 2H), 7.40–7.44 (m, 1H), 7.50–7.54 (m, 1H), 7.71–7.73 (m, 2H),7.82–7.86 (m, 1H), 7.94–7.96 (m, 2H); 13C-NMR (CDCl3) δ (ppm): 67.38 (d, 2JC-F = 20.8 Hz), 81.97 (d, 1JC-F = 170.50 Hz), 114.43, 117.08, 121.96, 122.01, 128.13, 130.17, 130.27, 132.10, 136.31, 136.95, 145.21, 158.95, 189.44; 19F-NMR (CDCl3) δ (ppm): −223.19 to −223.59 (m, 1F); IR (KBr) ν: 2928, 1659, 1604, 1491, 1310, 1262, 1213, 1178, 1048, 1027, 990, 883, 824, 778, 755, 666, 467 cm−1; HRMS (ESI) calcd. for C17H14BrFO2: [M + H]+ 349.0239; Found: 349.0232.
(E)-3-(3-(2-Fluoroethoxy)phenyl)-1-(3-methylphenyl)-prop-2-en-1-one (8ix). Pale yellow solid, Yield: 90%, m.p. 82–84 °C; 1H-NMR (CDCl3) δ (ppm): 2.44 (s, 3H), 4.27 (dt, 2H, 3JH-H = 27.8 Hz, 3JH-F = 3.9 Hz), 4.79 (dt, 2H, 3JH-H = 47.3 Hz, 2JH-F = 4.0 Hz), 6.98–7.00 (m,1H), 7.20 (s, 1H), 7.28–7.37 (m, 3H), 7.50–7.54 (m, 1H), 7.74–7.78 (m, 1H), 7.93–7.95 (m, 2H); 13C-NMR (CDCl3) δ (ppm): 21.82, 67.39 (d, 2JC-F = 20.4 Hz), 81.99 (d, 1JC-F = 171.90 Hz), 114.33, 116.82, 121.86, 122.71, 128.81, 129.50, 130.19, 135.70, 136.69, 143.88, 144.18, 158.95, 190.09; 19F-NMR (CDCl3) δ (ppm): −223.48 to −223.88 (m, 1F); IR (KBr) ν: 3055, 2920, 1658, 1600, 1576, 1494, 1439, 1372, 1311, 1205, 1182, 1096, 1078, 1055, 1030, 1016, 994, 884, 848 cm−1; HRMS (ESI) calcd. for C18H17FO2: [M + H]+ 285.1291 and [M + Na]+ 307.1110; Found: 285.1286 and 307.1104
(E)-3-(2-(2-Fluoroethoxy)phenyl)-1-phenylprop-2-en-1-one (9i). Pale yellow viscous liquid, Yield: 81%, 1H-NMR (CDCl3) δ (ppm): 4.33 (dt, 2H, 3JH-H = 3.9 Hz, 3JH-F = 27.5 Hz), 4.85 (dt, 2H, 3JH-H = 4.2 Hz, 2JH-F = 47.5 Hz), 6.93–6.95 (m, 1H), 7.02–7.06 (m, 1H), 7.37 (t, 1H, 3J = 8.3 Hz), 7.48–7.52 (m, 2H), 7.56–7.58 (m, 1H), 7.62–7.64 (m, 1H), 7.76–7.80 (m, 1H), 8.04–8.09 (m, 2H); 13C-NMR (CDCl3) δ (ppm): 67.68 (d, 2JC-F = 21.2 Hz), 81.87 (d, 1JC-F = 171.70 Hz), 112.36, 121.65, 123.68, 128.35, 128.71, 130.58, 131.70, 132.79, 133.09, 138.55, 140.49, 157.83, 191.21; 19F-NMR (CDCl3) δ (ppm): −223.86 to −223.46 (m, 1F); IR (KBr) ν: 2958, 2360, 1661, 1600, 1573, 1488, 1448, 1334, 1279, 1248, 1215, 1180, 1112, 1052, 1017, 927, 886, 753, 734, 694, 578 cm−1; HRMS (ESI) calcd. for C17H15FO2: [M + H]+ 271.1134 and [M + Na]+ 293.0954 ; Found: 271.1131 and 293.0945.
(E)-3-(2-(2-Fluoroethoxy)phenyl)-1-(4-methylphenyl)-prop-2-en-1-one (9ii). Yellow viscous liquid, Yield: 83%, m.p. 69–70 °C; 1H-NMR (CDCl3) δ (ppm): 2.43 (s, 3H), 4.33 (dt, 2H, 3JH-H = 4.2 Hz, 3JH-F = 27.5 Hz), 4.85 (dt, 2H, 3JH-H = 4.0 Hz, 2JH-F = 47.4 Hz), 6.93–6.95 (m, 1H), 7.02–7.06 (m, 1H), 7.29–7.31 (m, 2H), 7.34–7.38 (m, 1H), 7.62–7.64 (m, 1H), 7.77–7.80 (m, 1H), 7.95–7.97 (m, 2H), 8.04–8.08 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 21.81, 67.67 (d, 2JC-F = 20.6 Hz), 81.88(d, 1JC-F = 171.90 Hz), 112.34, 121.62, 123.73, 124.59, 128.84, 129.42, 130.55, 131.55, 135.98, 140.02, 143.59, 157.79, 190.69; 19F-NMR (CDCl3) δ (ppm): −223.82 to −223.43 (m, 1F); IR (KBr) ν: 2860, 2361, 1659, 1598, 1490, 1449, 1280, 1251, 1181, 1126, 1040, 930, 884, 742, 572, 474 cm−1; HRMS (ESI) calcd. for C18H17FO2: [M + H]+ 285.1291 and [M + Na]+ 307.1110 ; Found: 285.1288 and 307.1105.
(E)-1-(2,4-Dichlorophenyl)-3-(2-(2-fluoroethoxy)phenyl)prop-2-en-1-one (9iii). Yellow solid, Yield: 89%, m.p. 93–94 °C; 1H-NMR (CDCl3) δ (ppm): 4.28 (dt, 2H, 3JH-H = 4.3 Hz, 3JH-F = 27.7 Hz), 4.77 (dt, 2H, 3JH-H = 4.2 Hz, 2JH-F = 47.0 Hz), 6.92 (m, 1H), 7.01–7.04 (m, 1H), 7.24 (s,1H), 7.28–7.48 (m, 4H), 7.57–7.59 (m, 1H), 7.84 (d, 1H, 3J = 16.5 Hz); 13C-NMR (CDCl3) δ (ppm): 67.86 (d, 2JC-F = 20.6 Hz), 81.71 (d, 1JC-F = 171.80 Hz), 112.58, 112.74,123.90, 126.82, 127.30, 129.86, 130.28, 130.69, 132.40, 132.57, 136.90, 137.76, 142.11, 157.74, 193.13; 19F-NMR (CDCl3) δ (ppm): −223.85 to −223.46 (m, 1F); IR (KBr) ν: 2966, 1671, 1600, 1584, 1572, 1493, 1450, 1371, 1334, 1320, 1280, 1247, 1202, 1167, 1130, 1111, 1072, 1042, 1016, 987, 928, 886, 824, 790, 762, 745, 609, 575, 520, 454 cm−1; HRMS (ESI) calcd. for C17H13Cl2FO2 : [M + H]+ 339.0355; Found: 339.0344.
(E)-1-(4-Bromophenyl)-3-(2-(2-fluoroethoxy)phenyl)prop-2-en-1-one (9v). Pale yellow solid, Yield: 78%, m.p. 84–86 °C; 1H-NMR (CDCl3) δ (ppm): 4.33 (dt, 2H, 3JH-H = 6.2 Hz, 3JH-F = 23.0 Hz), 4.86 (dt, 2H, 3JH-H = 3.7 Hz, 2JH-F = 48.5 Hz), 6.93–6.95 (m, 1H), 7.02–7.06 (m, 1H), 7.36–7.40 (m, 1H), 7.57–7.65 (m, 3H), 7.74–7.78 (m, 1H), 7.83–7.85 (m, 1H), 7.90–7.93 (m, 1H), 8.03–8.07 (m, 1H); 13C-NMR (CDCl3) δ (ppm): 67.60 (d, 2JC-F = 20.7 Hz), 81.86 (d, 1JC-F = 171.30 Hz), 112.34, 121.67, 123.16, 124.26, 129.88, 130.24, 130.95, 131.89, 131.99, 137.27, 141.12, 157.91, 190.08; 19F-NMR (CDCl3) δ (ppm): −223.51 to −223.91 (m, 1F); IR (KBr) ν: 2960, 1919, 1684, 1657, 1600, 1571, 1490, 1448, 1394, 1333, 1274, 1252, 1205, 1177, 1128, 1064, 1008, 983, 930, 884, 822, 740, 667, 608, 580, 540, 468 cm−1; HRMS (ESI) calcd. for C17H14BrFO2: [M + H]+ 349.0239 and [M + Na]+ 371.0053; Found: 349.0230 and 371.0044.
(E)-3-(2-(2-Fluoroethoxy)phenyl)-1-(4-fluorophenyl)prop-2-en-1-one (9vii). Yellow solid, Yield: 71%, m.p. 44–45 °C; 1H-NMR (CDCl3) δ (ppm): 4.33 (dt, 2H, 3JH-H = 4.3 Hz, 3JH-F = 27.6 Hz), 4.86 (dt, 2H, 3JH-H = 3.9 Hz, 2JH-F = 47.5 Hz), 6.93–6.95 (m, 1H), 7.02–7.06 (m, 1H), 7.10–7.19 (m, 2H), 7.36–7.40 (m, 1H), 7.60–7.62 (m, 1H), 7.76–7.80 (m, 1H), 8.03–8.10 (m, 2H); 13C-NMR (CDCl3) δ (ppm): 67.66 (d, 2JC-F = 20.6 Hz), 81.87 (d, 1JC-F = 172.4), 112.35, 115.69, 115.90, 121.67, 123.34, 130.88, 130.96, 131.05, 131.24, 131.33, 131.79, 140.79, 189.77; 19F-NMR (CDCl3) δ (ppm): −105.71 to −105.78 (m, 1H) and −223.46 to −223.86 (m, 1H); HRMS (ESI) calcd. for C17H14F2O2: [M + H]+ 289.1040 and [M + Na]+ 311.0860; Found: 289.1025 and 311.0843.
(E)-3-(2-(2-Fluoroethoxy)phenyl)-1-(2-methylphenyl)-prop-2-en-1-one (9viii). Pale yellow viscous liquid, Yield: 85%, 1H-NMR (CDCl3) δ (ppm): 2.46 (s, 3H), 4.27 (dt, 2H, 3JH-H = 4.0 Hz, 3JH-F = 27.1 Hz), 4.75 (dt, 2H, 3JH-H = 4.1 Hz, 2JH-F = 48.1 Hz), 6.90–6.92 (m, 1H), 7.00–7.04 (m, 1H), 7.25–7.29 (m, 3H), 7.34–7.39 (m, 2H), 7.52–7.54 (m, 1H), 7.58–7.60 (m, 1H), 7.83 (d, 1H, 3J =16.4 Hz); 13C-NMR (CDCl3) δ (ppm): 20.49, 67.79 (d, 2JC-F = 20.40 Hz), 81.75 (d, 1JC-F = 172.6 Hz), 112.48, 121.67, 124.27, 125.54, 127.70, 128.43, 129.53, 130.52, 131.39, 131.92, 137.16, 139.34, 141.36, 15s7.54, 197.15; 19F-NMR (CDCl3) δ (ppm): −223.53 to −223.93 (m, 1F); IR (KBr) ν: 3707, 3314, 2869, 1636, 1600, 1489, 1459, 1390, 1310, 1276, 1245, 1168, 1112, 1064, 994, 922, 882, 748, 607, 458 cm−1; HRMS (ESI) calcd. for C18H17FO2: [M + H]+ 285.1291 and [M + Na]+ 307.1110; Found: 285.1286 and 307.1102.