Antiproliferative Phenothiazine Hybrids as Novel Apoptosis Inducers against MCF-7 Breast Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
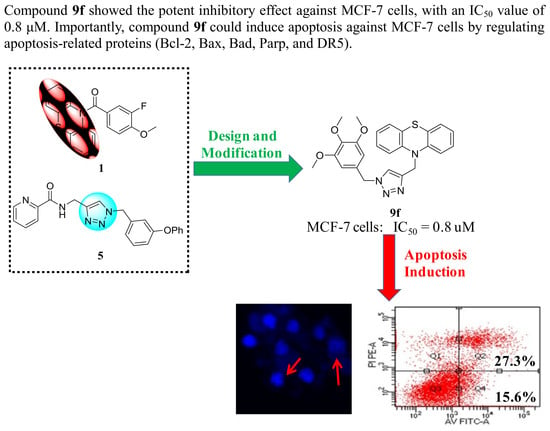
2.2. Antiproliferative Activity and SARs
2.3. Compound 9f Induced Morphological Changes of Mcf-7 Cells
2.4. Compound 9f Induced Apoptosis of Mcf-7 Cells
2.5. Compound 9f Related Apoptosis-Related Proteins
3. Experimental Section
3.1. Chemistry
3.1.1. Phenothiazine Derivatives 8a–8b
3.1.2. General Method to Synthesize Phenothiazine-1,2,3-Triazole Hybrids 9a–9k
3.2. Biology
3.2.1. Antiproliferative Activity
3.2.2. Apoptosis Analysis with Hoechst 33258 Staining
3.2.3. Apoptosis Analysis with Western Blot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA-Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Caruso, D.; Tomao, S.; Rossi, L.; Zaccarelli, E.; Tomao, F. Triple-negative breast cancer: Investigating potential molecular therapeutic target. Expert Opin. Ther. Targets 2015, 19, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Bai, L.Y.; Tsai, M.H.; Chu, P.C.; Chiu, C.F.; Chen, M.Y.; Chiu, S.J.; Chiang, J.H.; Weng, J.R. Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents—A drug repurposing strategy. Sci. Rep. 2016, 6, 27540. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M. 6-Substituted 9-fluoroquino [3,2-b]benzo[1,4]thiazines display strong antiproliferative and antitumor properties. Eur. J. Med. Chem. 2015, 89, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Maddila, S.; Naicker, K.; Momin, M.I.K.; Rana, S.; Gorle, S.; Maddila, S.; Yalagala, K.; Singh, M.; Koorbanally, N.A.; Jonnalagadda, S.B. Novel 2-(1-(substitutedbenzyl)-1H-tetrazol-5-yl)-3-phenylacrylonitrile derivatives: Synthesis, in vitro antitumor activity and computational studies. Med. Chem. Res. 2016, 25, 283–291. [Google Scholar] [CrossRef]
- Ghinet, A.; Moise, I.-M.; Rigo, B.; Homerin, G.; Farce, A.; Dubois, J.; Bîcu, E. Studies on phenothiazines: New microtubule-interacting compounds with phenothiazine A-ring as potent antineoplastic agents. Bioorg. Med. Chem. 2016, 24, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Prinz, H.; Ridder, A.K.; Vogel, K.; Böhm, K.J.; Ivanov, I.; Ghasemi, J.B.; Aghaee, E.; Müller, K. N-Heterocyclic (4-Phenylpiperazin-1-yl)methanones Derived from Phenoxazine and Phenothiazine as Highly Potent Inhibitors of Tubulin Polymerization. J. Med. Chem. 2017, 60, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Verones, V.; Flouquet, N.; Lecoeur, M.; Lemoine, A.; Farce, A.; Baldeyrou, B.; Mahieu, C.; Wattez, N.; Lansiaux, A.; Goossens, J.-F.; et al. Synthesis, antiproliferative activity and tubulin targeting effect of acridinone and dioxophenothiazine derivatives. Eur. J. Med. Chem. 2013, 59, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Shankaraiah, N.; Devaiah, V.; Laxma Reddy, K.; Juvekar, A.; Sen, S.; Kurian, N.; Zingde, S. Synthesis of 1,2,3-triazole-linked pyrrolobenzodiazepine conjugates employing ‘click’ chemistry: DNA-binding affinity and anticancer activity. Bioorg. Med. Chem. Lett. 2008, 18, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.S.; Kaur, T.; Saxena, A.K. 1,2,3-Triazole tethered β-lactam-Chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Fu, D.-J.; Yue, X.-X.; Liu, Y.-C.; Song, J.; Sun, H.-H.; Liu, H.-M.; Zhang, Y.-B. Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents. Molecules 2016, 21, 653. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Yao, Q.; Yu, S.; Gong, P.; Qin, M. Synthesis and Antitumor Activity of Triazole-Containing Sorafenib Analogs. Molecules 2017, 22, 1759. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Xi, C. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.M.; Anhäuser, L.; Rentmeister, A. Modifying the 5′-Cap for Click Reactions of Eukaryotic mRNA and To Tune Translation Efficiency in Living Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 10899–10903. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.J.; Zhang, S.Y.; Liu, Y.C.; Zhang, L.; Liu, J.J.; Song, J.; Zhao, R.H.; Li, F.; Sun, H.H.; Liu, H.M. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate-chalcone derivates. Bioorg. Med. Chem. Lett. 2016, 26, 3918–3922. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Martinez, G.J.; Payet, C.A.; Barraclough, J.Y.; Celermajer, D.S.; Bursill, C.; Patel, S. Colchicine Therapy in Acute Coronary Syndrome Patients acts on Caspase-1 to Suppress NLRP3 Inflammasome Monocyte Activation. Clin. Sci. 2016, 130, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Miyakawa, K.; Denda, T.; Mizukami, T.; Horie, Y.; Izawa, N.; Hirakawa, M.; Ogura, T.; Tsuda, T.; Yu, S. Early morphological change for predicting outcome in metastatic colorectal cancer after regorafenib. Oncotarget 2017, 8, 110530–110539. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.M.F.; Salvador, J.A.R.; Marín, S.; Cascante, M. Synthesis and anticancer activity of novel fluorinated asiatic acid derivatives. Eur. J. Med. Chem. 2016, 114, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Inoueyamauchi, A.; Jeng, P.S.; Kim, K.; Chen, H.C.; Han, S.; Ganesan, Y.T.; Ishizawa, K.; Jebiwott, S.; Dong, Y.; Pietanza, M.C. Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat. Commn. 2017, 8, 16078. [Google Scholar] [CrossRef] [PubMed]
- Kunciw, D.L.; Liechty, J.J.; Mitchell, M.O.; Wan, B.J.; Franzblau, S.G. Structural requirements for the antitubercular quaternized triflupromazine pharmacophore. Bioorg. Med. Chem. Lett. 2012, 22, 5679–5680. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |









| Compound | IC50 (μM) a | ||
|---|---|---|---|
| MDA-MB-231 | MDA-MB-468 | MCF-7 | |
| 8a | >100 | >100 | >100 |
| 8b | >100 | >100 | >100 |
| 9a | 9.0 ± 0.6 | 15.7 ± 0.3 | 8.6 ± 1.1 |
| 9b | 11.4 ± 0.2 | 5.5 ± 1.7 | 8.8 ± 2.0 |
| 9c | 14.2 ± 1.3 | 9.0 ± 0.9 | 11.1 ± 0.5 |
| 9d | 2.0 ± 0.2 | 1.6 ± 0.3 | 7.3 ± 1.1 |
| 9e | 10.5 ± 1.0 | 8.4 ± 0.8 | 7.2 ± 0.2 |
| 9f | 1.7 ± 0.1 | 1.2 ± 0.2 | 0.8 ± 0.1 |
| 9g | 56.3 ± 0.8 | 46.4 ± 2.8 | >100 |
| 9h | 39.1 ± 0.6 | 43.3 ± 0.7 | 29.6 ± 1.3 |
| 9i | 11.3 ± 2.6 | 11.2 ± 0.8 | 14.2 ± 1.7 |
| 9j | 17.4 ± 1.4 | 17.6 ± 2.4 | 15.3 ± 0.4 |
| 9k | 17.3 ± 0.9 | 14.3 ± 0.8 | 16.4 ± 1.1 |
| 5-Fu | 10.8 ± 0.3 | 7.5 ± 0.2 | 12.7 ± 0.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-X.; Guo, J.-M.; Zhang, T.-T.; Lin, H.-J.; Qi, N.-S.; Li, Z.-G.; Zhou, J.-C.; Zhang, Z.-Z. Antiproliferative Phenothiazine Hybrids as Novel Apoptosis Inducers against MCF-7 Breast Cancer. Molecules 2018, 23, 1288. https://doi.org/10.3390/molecules23061288
Zhang J-X, Guo J-M, Zhang T-T, Lin H-J, Qi N-S, Li Z-G, Zhou J-C, Zhang Z-Z. Antiproliferative Phenothiazine Hybrids as Novel Apoptosis Inducers against MCF-7 Breast Cancer. Molecules. 2018; 23(6):1288. https://doi.org/10.3390/molecules23061288
Chicago/Turabian StyleZhang, Jun-Xia, Jiao-Mei Guo, Ting-Ting Zhang, Hong-Jun Lin, Nai-Song Qi, Zhen-Guo Li, Ji-Chun Zhou, and Zhen-Zhong Zhang. 2018. "Antiproliferative Phenothiazine Hybrids as Novel Apoptosis Inducers against MCF-7 Breast Cancer" Molecules 23, no. 6: 1288. https://doi.org/10.3390/molecules23061288
APA StyleZhang, J. -X., Guo, J. -M., Zhang, T. -T., Lin, H. -J., Qi, N. -S., Li, Z. -G., Zhou, J. -C., & Zhang, Z. -Z. (2018). Antiproliferative Phenothiazine Hybrids as Novel Apoptosis Inducers against MCF-7 Breast Cancer. Molecules, 23(6), 1288. https://doi.org/10.3390/molecules23061288