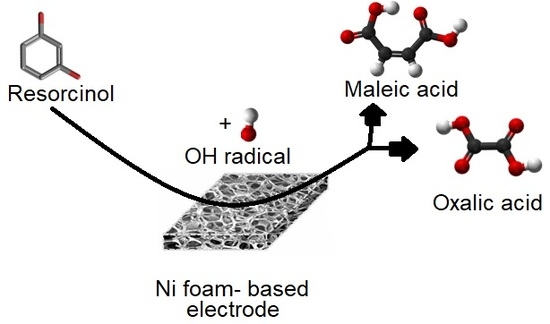
Electrodegradation of Resorcinol on Pure and Catalyst-Modified Ni Foam Anodes, Studied under Alkaline and Neutral pH Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. EDX and SEM Characterizations of Nickel Foam and Pd-Modified Nickel Foam Electrodes
3.2. Electrochemical Characteristics of Resorcinol Oxidation Reaction
4. Conclusions
Autor Contributions
Funding
Conflicts of Interest
References
- Hahn, S.; Kielhorn, J.; Koppenhöfer, J.; Wibbertmann, A.; Mangelsdorf, I. Concise International Chemical Assessment Document 71; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Porras, S.P.; Hartonen, M.; Ylinen, K.; Tornaeus, J.; Tuomi, T.; Santonen, T. Environmental and occupational exposure to resorcinol in Finland. Toxicol. Lett. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Korbahti, B.K.; Demirbuken, P. Electrochemical oxidation of resorcinol in aqueous medium using boron-doped diamond anode: Reaction kinetics and process optimization with response surface methodology. Front. Chem. 2017, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, D.; Palanivelu, K.; Mohan, N. Electrochemical oxidation of resorcinol for wastewater treatment—A kinetic study. Indian J. Chem. Technol. 2003, 10, 396–401. [Google Scholar]
- Nady, H.; El-Rabieci, M.M.; Abd El-Hafez, G.M. Electrochemical oxidation behavior of some hazardous phenolic compounds in acidic solution. Egypt. J. Pet. 2017, 26, 669–678. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, Y.; Liu, W.; Chang, L. Investigation on electro-catalytic oxidation properties of carbon nanotube-Ce-modified PbO2 electrode and its application for degradation of m-nitrophenol. Arab. J. Chem. 2014, in press. [Google Scholar] [CrossRef]
- Rajkumar, D.; Palanivelu, K. Electrochemical treatment of industrial wastewater. J. Hazard. Mater. 2004, B113, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Zhang, Y.; Han, W.; Sun, X.; Li, J.; Wang, L. Preparation of Ti/PbO2–Sn anodes for electrochemical degradation of phenol. J. Electroanal. Chem. 2013, 689, 193–200. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Liu, W.; Tu, Y.; Zhang, Y.; Han, W.; Wang, L. Electrochemical degradation of nitrobenzene by anodic oxidation on the constructed TiO2-NTs/SnO2-Sb/PbO2 electrode. Chemosphere 2014, 113, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Pierozynski, B.; Mikolajczyk, T.; Piotrowska, G. Electrooxidation of phenol at palladium-based catalyst materials in alkaline solution. Int. J. Electrochem. Sci. 2015, 10, 2088–2097. [Google Scholar]
- Pierozynski, B.; Mikolajczyk, T.; Kowalski, I.M. Hydrogen evolution at catalytically-modified nickel foam in alkaline solution. J. Power Sources 2014, 271, 231–238. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy, Emphasizing Solid Materials and Systems; John Wiley: New York, NY, USA, 1987; ISBN 0471831220. [Google Scholar]
- Mendham, J.; Denney, R.C.; Barnes, J.D.; Thomas, M. Vogel’s Textbook of Quantitative Chemical Analysis, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2000; ISBN 13 2900582226288. [Google Scholar]
- Abdulkadir, M.Q. Proceeding bromometric phenol assay without starch indicator. Iraqi J. Pharm. Sci. 2009, 18, 72–77. [Google Scholar]
- Van Drunen, J.T.; Napporn, W.; Kokoh, B.; Jerkiewicz, G. Electrochemical oxidation of isopropanol using a nickel foam electrode. J. Electroanal. Chem. 2014, 716, 120–128. [Google Scholar] [CrossRef]
- Van Drunen, J.; Kinkead, B.; Wang, M.C.P.; Sourty, E.; Gates, B.D.; Jerkiewicz, G. Comprehensive structural, surface-chemical and electrochemical characterization of nickel-based metallic foams. ASC Appl. Mater. Interfaces 2013, 5, 6712–6722. [Google Scholar] [CrossRef] [PubMed]
- Grden, M.; Czerwinski, A. Surface science and electrochemical analysis of nickel foams. J. Solid State Electrochem. 2008, 12, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Verlato, E.; Cattarin, S.; Comisso, N.; Gambirasi, A.; Musiani, M.; Vazquez-Gomez, L. Preparation of Pd-modified Ni foam electrodes and their use as anodes for the oxidation of alcohols in basic media. Electrocatalysis 2012, 3, 48–58. [Google Scholar] [CrossRef]
- Henning, S.; Herranz, J.; Gasteiger, H.A. Bulk-palladium and palladium-on-gold electrocatalysts for the oxidation of hydrogen in alkaline electrolyte. J. Electrochem. Soc. 2015, 162, F178–F189. [Google Scholar] [CrossRef]
- Pierozynski, B.; Morin, S.; Conway, B.E. Influence of adsorption of guanidonium cations on H upd at Pt(hkl) surfaces: Lattice-specific anion-mimetic effects. J. Electroanal. Chem. 1999, 7, 30–42. [Google Scholar] [CrossRef]
- Bejerano, T.; Forgacs, C.; Gileadi, E. Selective inhibition of electrode reactions by organic compounds: I. The inhibition of Br2 and I2 evolution on platinum by phenol. J. Electroanal. Chem. 1970, 27, 69–79. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| Elements | Measurements | A * | B * | C * | D * |
|---|---|---|---|---|---|
| Ni/% | 1 | 94.05 | 77.50 | 87.86 | 63.80 |
| 2 | 93.87 | 70.86 | 85.56 | 73.36 | |
| 3 | 95.01 | 72.36 | 88.73 | 74.27 | |
| C/% | 1 | 5.39 | 3.88 | 9.30 | 5.70 |
| 2 | 5.59 | 3.36 | 9.31 | 5.61 | |
| 3 | 4.83 | 3.97 | 8.24 | 5.22 | |
| O/% | 1 | 0.56 | 0.78 | 2.83 | 2.40 |
| 2 | 0.54 | 0.45 | 5.13 | 2.51 | |
| 3 | 0.16 | 0.56 | 3.03 | 2.40 | |
| Pd/% | 1 | — | 17.00 | — | 28.10 |
| 2 | — | 25.33 | — | 18.52 | |
| 3 | — | 23.11 | — | 18.11 |
| E/mV | RAds/Ω g | CAds/µF g−1 sφ1−1 | RF/Ω g | Cdl/µF g−1 sφ2−1 |
|---|---|---|---|---|
| Electrode I | ||||
| 1100 | 0.19 ± 0.01 | 64,157 ± 894 | 10.38 ± 0.67 | 91,865 ± 8543 |
| 1200 | 0.11 ± 0.01 | 54,892 ± 1762 | 13.63 ± 0.68 | 65,533 ± 8061 |
| 1300 | 0.12 ± 0.01 | 54,762 ± 679 | 21.68 ± 1.94 | 52,718 ± 7117 |
| Electrode II | ||||
| 1100 | 0.20 ± 0.01 | 300,890 ± 1866 | 3.76 ± 0.05 | 245,411 ± 1006 |
| 1200 | 0.06 ± 0.00 | 142,843 ± 1243 | 11.43 ± 0.34 | 201,937 ± 666 |
| 1300 | 0.07 ± 0.00 | 138,788 ± 1485 | 11.63 ± 0.89 | 92,558 ± 324 |
| 1400 | 0.44 ± 0.09 | 87,120 ± 13,168 | 29.11 ± 2.10 | 31,699 ± 3828 |
| Electrode III | ||||
| 1100 | 0.21 ± 0.01 | 35,251 ± 325 | 9.32 ± 0.05 | 32,625 ± 108 |
| 1200 | 0.07 ± 0.00 | 32,563 ± 243 | 11.62 ± 0.31 | 29,328 ± 356 |
| 1300 | 0.08 ± 0.00 | 30,890 ± 177 | 11.45 ± 0.89 | 24,124 ± 89 |
| 1400 | 0.32 ± 0.07 | 28,545 ± 9512 | 20.67 ± 1.72 | 10,359 ± 2576 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikolajczyk, T.; Pierozynski, B.; Smoczynski, L.; Wiczkowski, W. Electrodegradation of Resorcinol on Pure and Catalyst-Modified Ni Foam Anodes, Studied under Alkaline and Neutral pH Conditions. Molecules 2018, 23, 1293. https://doi.org/10.3390/molecules23061293
Mikolajczyk T, Pierozynski B, Smoczynski L, Wiczkowski W. Electrodegradation of Resorcinol on Pure and Catalyst-Modified Ni Foam Anodes, Studied under Alkaline and Neutral pH Conditions. Molecules. 2018; 23(6):1293. https://doi.org/10.3390/molecules23061293
Chicago/Turabian StyleMikolajczyk, Tomasz, Boguslaw Pierozynski, Lech Smoczynski, and Wieslaw Wiczkowski. 2018. "Electrodegradation of Resorcinol on Pure and Catalyst-Modified Ni Foam Anodes, Studied under Alkaline and Neutral pH Conditions" Molecules 23, no. 6: 1293. https://doi.org/10.3390/molecules23061293
APA StyleMikolajczyk, T., Pierozynski, B., Smoczynski, L., & Wiczkowski, W. (2018). Electrodegradation of Resorcinol on Pure and Catalyst-Modified Ni Foam Anodes, Studied under Alkaline and Neutral pH Conditions. Molecules, 23(6), 1293. https://doi.org/10.3390/molecules23061293