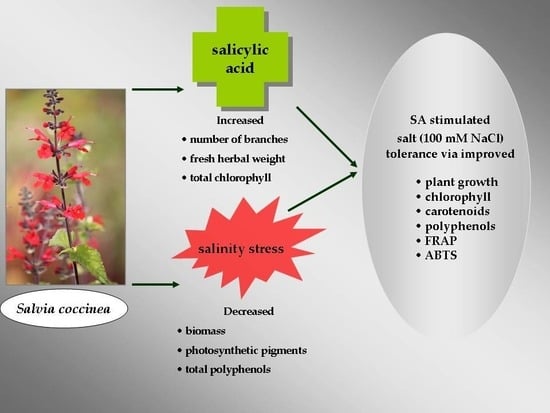
Changes in Photosynthetic Pigments, Total Phenolic Content, and Antioxidant Activity of Salvia coccinea Buc’hoz Ex Etl. Induced by Exogenous Salicylic Acid and Soil Salinity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Attributes
2.2. Photosynthetic Pigment Contents
2.3. Total Polyphenol Content and Antioxidant Activity
3. Materials and Methods
3.1. Plant Culture, Treatment, and Measurement of Growth Parameters
3.2. Photosynthetic Pigment Determination
3.3. Determination of Total Polyphenol Content and Antioxidant Activity
3.3.1. Preparation of Plant Extracts
3.3.2. Total Polyphenol Content
3.3.3. Determination of Ferric-Reducing Antioxidant Power (FRAP)
3.3.4. Determination of Free Radical-Scavenging Ability Using a Stable ABTS Radical Cation
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pop (Cuceu), A.-V.; Tofană, M.; Socaci, S.A.; Nagy, M.; Borş, M.-D.; Salanţă, L.; Vlaic, R. Studies on total polyphenols content and antioxidant activity of methanolic extracts from selected Salvia species. Bull. UASVM Food Sci. Technol. 2015, 72, 86–90. [Google Scholar] [CrossRef]
- Yadav, A.; Joshi, A.; Kothari, S.L.; Kachhwaha, S.; Purohit, S. Medicinal, nutritional and industrial applications of Salvia species: A revisit. Int. J. Pharm. Sci. Rev. Res. 2017, 43, 27–37. [Google Scholar]
- Lamien-Meda, A.; Nell, M.; Lohwasser, U.; Börner, A.; Franz, C.; Novak, J. Investigation of antioxidant and rosmarinic acid variation in the sage collection of the genebank in gatersleben. J. Agric. Food Chem. 2010, 58, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Chemistry, pharmacology and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease and cancer. Glob. J. Med. Res. 2013, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Muráriková, A.; Kaffková, K.; Raab, S.; Neugebauerová, J. Evaluation of content of phenolics in Salvia species cultivated in South Moravian Region. Acta Fac. Pharm. Univ. Comen. 2015, 62, 18–22. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Rauter, A.P.; Dias, C.; Martins, A.; Branco, I.; Neng, N.R.; Nogueira, J.M.; Goulart, M.; Silva, F.V.M.; Justino, J.; Trevitt, C.; et al. Non-toxic Salvia sclareoides Brot. extracts as a source of functional food ingredients: phenolic profile, antioxidant activity and prion binding properties. Food Chem. 2012, 132, 1930–1935. [Google Scholar] [CrossRef]
- Esan, A.M.; Masisi, K.; Dada, F.A.; Olaiya, C.O. Comparative effects of indole acetic acid and salicylic acid onoxidative stress marker and antioxidant potential of okra (Abelmoschus esculentus) fruit under salinity stress. Sci. Hortic. 2017, 216, 278–283. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Notes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef] [PubMed]
- Simaei, M.; Khavari-Nejad, R.A.; Bernard, F. Exogenous application of salicylic acid and nitric oxide on the ionic contents and enzymatic activities in NaCl-stressed soybean plants. Am. J. Plant. Sci. 2012, 3, 1495–1503. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Lee, H.; León, J.; Raskin, I. Biosynthesis and metabolism of salicylic acid. Proc. Natl. Acad. Sci. USA 1995, 92, 4076–4079. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Dang, X.; Dong, J. Hydrogen peroxide and nitric oxide are involved in salicylic acid-induced salvianolic acid B production in Salvia miltiorrhiza cell cultures. Molecules 2014, 19, 5913–5924. [Google Scholar] [CrossRef] [PubMed]
- Elwan, M.W.M.; El-Hamahmy, M.A.M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009, 122, 521–526. [Google Scholar] [CrossRef]
- Dong, C.-J.; Wang, X.-L.; Shang, Q.-M. Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci. Hortic. 2011, 129, 629–636. [Google Scholar] [CrossRef]
- Asmaa, R.A.E.H.; Ahmed, M.A.; Karima, M.G.E.D.; Magda, A.S.; Hoda, M.E. Role of salicylic acid to improve physiological characters and bio-chemical markers of soybean (Glycine max L.) under sea salt stress. Int. J. Environ. Res. 2017, 11, 547–556. [Google Scholar] [CrossRef]
- Bideshki, A.; Arvin, M.J. Effect of salicylic acid (SA) and drought stress on growth bulb yield and allicin content of garlic (Allium sativum) the field. J. Plant Econ. Physiol. 2010, 2, 73–79. [Google Scholar]
- Bagherifard, A.; Bagheri, A.; Sabourifard, H.; Bagherifard, G.; Najar, M. The effect of salicylic acid on some morphological and biochemistry parameters under salt stress in Herb Artichoke (Cynara scolymus L.). Res. J. Fish. Hydrobiol. 2015, 10, 745–750. [Google Scholar]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Sahar, K.; Amin, B.; Taher, N.M. The salicylic acid effect on the Salvia officianlis L. sugar, protein and proline contents under salinity (NaCl) stress. J. Stress Physiol. Biochem. 2011, 7, 80–87. [Google Scholar]
- Stratu, A.; Lobiuc, A. The influence of lead on seed germination and seedlings growth of Ocimum basilicum L. and Salvia coccinea Buchoz ex Etl. species. Analele Stiin. Ale Univ. 2015, 61, 39–47. [Google Scholar]
- Ljubojević, M.; Ognjanov, V.; Maksimović, I.; Čukanović, J.; Dulić, J.; Szabò, Z.; Szabò, E. Effects of hydrogel on growth and visual damage of ornamental Salvia species exposed to salinity. Clean Soil Air Water 2017, 45, 1600128. [Google Scholar] [CrossRef]
- Radhika, J.; Pazhanithambi, G.; Brindha, P. Antioxidant and free radical scavenging property of Salvia coccinea Buc’Hoz. Biomedicine 2009, 29, 52–55. [Google Scholar]
- Yadav, S.; Mukundan, U. In vitro antioxidant properties of Salvia coccinea Buc’hoz ex Etl. and Salvia officinalis L. Indian J. Fundam Appl. Life Sci. 2011, 1, 232–238. [Google Scholar]
- Bilgin, M.; Sahin, S.; Dramur, M.U.; Sevgili, L.M. Obtaining scarlet sage (Salvia coccinea) extract through homogenizer- and ultrasound-assisted extraction methods. Chem. Eng. Comm. 2013, 200, 1197–1209. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.J.; Hawa, Z.E. Interactive effect of salicylic acid on some physiological features and antioxidant enzymes activity in ginger (Zingiber. officinale Roscoe). Molecules 2013, 18, 5965–5979. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.K. Salicylic acid: Role in plant physiology and stress tolerance. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 217–239. [Google Scholar]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Manaa, A.; Gharbi, E.; Mimouni, H.; Wasti, S.; Aschi-Smiti, S.; Lutts, S.; Ahmed, H.B.A. Simultaneous application of salicylic acid and calcium improves salt tolerance in two contrasting tomato (Solanum lycopersicum) cultivars. S. Afr. J. Bot. 2014, 95, 32–39. [Google Scholar] [CrossRef]
- Salachna, P.; Piechocki, R.; Zawadzińska, A.; Wośkowiak, A. Response of speckled spur-flower to salinity stress and salicylic acid treatment. J. Ecol. Eng. 2015, 16, 68–75. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Gengmao, Z.; Quanmei, S.; Yu, H.; Shihui, L.; Changhai, W. The physiological and biochemical responses of a medicinal plant (Salvia miltiorrhiza L.) to stress caused by various concentrations of NaCl. PLoS ONE 2014, 9, e89624. [Google Scholar] [CrossRef] [PubMed]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Tehami, W.; Kherraf, A.; Boufeldja, W.; Mahmoud, M.; Ghislaine, A.; Abbouni, B.; Benali, M. Determination of primary and functional metabolites of Salvia argentea and evaluation of its leaves and roots antioxidant activity. Der Pharma Chem. 2016, 8, 1–6. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Taârit, M.B.; Msaada, K.; Hosni, K.; Marzouk, B. Fatty acids, phenolic changes and antioxidant activity of clary sage (Salvia sclarea L.) rosette leaves grown under saline conditions. Ind. Crops Prod. 2012, 38, 58–63. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.-H.; Chung, I.-M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.; Zhang, X.; Xu, L.; Cao, J.; Jiang, W. The effect of exogenous salicylic acid on antioxidant activity, bioactive compounds and antioxidant system in apricot fruit. Sci. Hortic. 2015, 181, 113–120. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Andrys, D.; Kulpa, D.; Grzeszczuk, M.; Bihun, M.; Dobrowolska, A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated in vitro. Folia Hort. 2017, 29, 161–180. [Google Scholar] [CrossRef]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Wan Aida, W.M.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427–1435. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzeszczuk, M.; Salachna, P.; Meller, E. Changes in Photosynthetic Pigments, Total Phenolic Content, and Antioxidant Activity of Salvia coccinea Buc’hoz Ex Etl. Induced by Exogenous Salicylic Acid and Soil Salinity. Molecules 2018, 23, 1296. https://doi.org/10.3390/molecules23061296
Grzeszczuk M, Salachna P, Meller E. Changes in Photosynthetic Pigments, Total Phenolic Content, and Antioxidant Activity of Salvia coccinea Buc’hoz Ex Etl. Induced by Exogenous Salicylic Acid and Soil Salinity. Molecules. 2018; 23(6):1296. https://doi.org/10.3390/molecules23061296
Chicago/Turabian StyleGrzeszczuk, Monika, Piotr Salachna, and Edward Meller. 2018. "Changes in Photosynthetic Pigments, Total Phenolic Content, and Antioxidant Activity of Salvia coccinea Buc’hoz Ex Etl. Induced by Exogenous Salicylic Acid and Soil Salinity" Molecules 23, no. 6: 1296. https://doi.org/10.3390/molecules23061296
APA StyleGrzeszczuk, M., Salachna, P., & Meller, E. (2018). Changes in Photosynthetic Pigments, Total Phenolic Content, and Antioxidant Activity of Salvia coccinea Buc’hoz Ex Etl. Induced by Exogenous Salicylic Acid and Soil Salinity. Molecules, 23(6), 1296. https://doi.org/10.3390/molecules23061296