Anthocyanins of Coloured Wheat Genotypes in Specific Response to SalStress
Abstract
:1. Introduction
2. Results
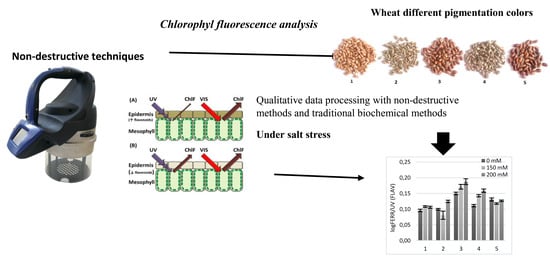
2.1. SFR, ANTH, FLAV and MFI
2.2. Anthocyanins Content
2.3. Proline Content
2.4. Lipid Peroxidation Assay
2.5. Plant Biomass
2.6. Na+ and K+ Level
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Growth Conditions
4.3. Growth Parameters
4.4. Chlorophyll Fluorescence Records and Analyses Using the Fluorescence Excitation Ratio Method
4.5. Proline Assay
4.6. Lipid Peroxidation (LP) Assay
4.7. Anthocyanin Estimation
4.8. Na+ and K+ Accumulation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibbs, H.K.; Salmon, J.M. Mapping the world’s degraded lands. Appl. Geogr. 2015, 57, 12–21. [Google Scholar] [CrossRef]
- Geist, H. The Causes and Progression of Desertification; Routledge: London, UK, 2017. [Google Scholar]
- Lachhab, I.; Louahlia, S.; Laamarti, M.; Hammani, K. Effet d’un stress salin sur la germination et l’activité enzymatique chez deux génotypes de Medicago sativa. Int. J. Innov. Appl. Stud. 2013, 3, 511–516. [Google Scholar]
- FAO. FAOSTAT. Online Statistical Database. 2016. Available online: http://faostat.fao. org/ (accessed on 30 July 2016).
- Minhas, P.S. Edaphic stresses: Concerns and opportunities for management. In Abiotic Stress Management for Resilient Agriculture; Minhas, P.S., Rane, J., Pasala, R.K., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Li, X.N.; Zhu, X.C.; Liu, S.Q.; Song, F.B.; Liu, F.L.; Wang, Y.; Qi, X.N.; Wang, F.H.; Zuo, Z.Y.; et al. Salt acclimation induced salt tolerance is enhancedby abscisic acid priming in wheat. Plant Soil Environ. 2017, 63, 307–314. [Google Scholar]
- Mbarki, S.; Cerdà, A.; Zivcak, M.; Brestic, M.; Rabhi, M.; Mezni, M.; Abdelly, C.; Pascual, J.A. Alfalfa crops amended with MSW compost can compensate the effect of salty water irrigation depending on the soil texture. Process Saf. Environ. Prot. 2018, 115, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, R.S.; Khalilzadeh, R.; Jalilian, J. Effects of biofertilizers and cycocel on some physiological and biochemical traits of wheat (Triticum aestivum L.) under salinity stress. Arch. Agron. Soil Sci. 2016, 63, 308–318. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Midaoui, M.; Benbella, M.; Aït Houssa, A.; Ibriz, M.; Talouizte, A. Contribution to the study of some mechanisms of adaptation to salinity in cultivated sunflower (Helianthus annuus L.). Revue HTE 2007, 136, 29–34. [Google Scholar]
- Hamed, K.B.; Chibani, F.; Abdelly, C.; Magne, C. Growth, sodium uptake and antioxidant responses of coastal plants differing in their ecological status under increasing salinity. Biologia 2014, 69, 193–201. [Google Scholar] [Green Version]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Dardanelli, M.S.; Fernández de Córdoba, F.J.; Espuny, M.R.; Rodríguez Carvajal, M.A.; Soria Díaz, M.E.; Gil Serrano, A.M.; Yaacov, O.; Megías, M. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol. Biochem. 2008, 40, 2713–2721. [Google Scholar] [CrossRef]
- Daneshmand, F.; Arvin, M.J.; Kalantari, K.M. Physiological responses to NaCl stress in three wild species of potato in vitro. Acta Physiol. Plant 2010, 32, 91–101. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kontek, R.; Janas, K.M. Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol. Environ. Saf. 2009, 72, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Yoshida, K.; Nakagawa, A.; Kawai, T.; Tamura, H.; Goto, T. Commelinin, a highly associated metalloanthocyanin present in the blue flower petals of Commelina communis. Nature 1992, 358, 515–517. [Google Scholar]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Scientia Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Ngara, R.; Ndimba, R.; Jensen, J.B.; Jensen, O.N.; Ndimb, B. Identification and profiling of salinity stress-responsive proteins in Sorghum bicolor seedlings. J. Proteomics 2012, 75, 4139–4150. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Oyiga, B.C.; Sharma, R.C.; Shen, J.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Identification and characterization of salt tolerance of wheat germplasm using a multivariable screening approach. J. Agron. Crop Sci. 2016, 202, 472–485. [Google Scholar] [CrossRef]
- Kalhoro, N.A.; Rajpar, I.; Kalhoro, S.A.; Ali, A.; Raza, S.; Ahmed, M.; Kalhoro, F.A.; Ramzan, M.; Wahid, F. Effect of salts stress on the growth and yield of wheat (Triticum aestivum L.). Am. J. Plant Sci. 2016, 7, 2257–2271. [Google Scholar] [CrossRef]
- Zivcak, M.; Brückova, K.; Sytar, O.; Brestic, M.; Olsovska, K.; Allakhverdiev, S.I. Lettuce flavonoids screening and phenotyping by chlorophyll fluorescence excitation ratio. Planta 2017, 245, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Chutipaijit, S.; Chaum, S.; Sompornpailin, K. High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. spp. indica. Aust. J. Crop Sci. 2011, 5, 1191–1198. [Google Scholar]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Ghozlen, N.B.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B.; Bertrand, M.; Lemoine, Y. Changes in the photosynthetic pigments in bean leaves during the first photoperiod of greening and the subsequent dark-phase. Comparison between old (10-d-old) leaves and young (2-d-old) leaves. Photosynth. Res. 1998, 57, 203. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Dubey, R.S. Photosynthesis in plants under stressful conditions. In Hand Book Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; C.R.C. Press: New York, NY, USA, 2005; pp. 717–718. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycinebetaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. Composition and stability of anthocyanins in blue-grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Knievel, D.C.; Abdel-Aal, E.S.M.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 2013, 8, e62085. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.; Khan, M.; Mahboob, W.; Khan, M.A.; Shereen, A.; Mujtaba, S.; Asad, A. Inconsistency in salt tolerance of some wheat (Triticum aestivium L.) genotypes evaluated under various growing environments. Pak. J. Bot. 2018, 50, 471–479. [Google Scholar]
- Matysik, J.; Alia, T.A.; Bhalu, B.A.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuin, T.A.; Shabala, S. Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 2007, 225, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Manivannan, P.; Kishorekumar, A.; Sridharan, R.; Panneerselvam, R. Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. S. Afr. J. Bot. 2007, 73, 190–195. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Koca, H.; Melike, B.; Özdemir, F.; Türkan, İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Brini, F. Comparison of an antioxidant system in tolerant and susceptible wheat seedlings in response to salt stress. Span. J. Agric. Res. 2018, 15, 0805. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Afzal, I.; Basra, S.M.; Ali, Z.; Ibrahim, A.M. Sodium exclusion is a reliable trait for the improvement of salinity tolerance in bread wheat. Arch. Agron. Soil Sci. 2018, 64, 272–284. [Google Scholar] [CrossRef]
- Cramer, G.R.; Läuchli, A.; Polito, V.S. Displacement of Ca2+ by Na+ from the plasmalemma of root cells: A primary response to salt stress? Plant Physiol. 1985, 79, 207–211. [Google Scholar] [CrossRef] [PubMed]
- El-Iklil, Y.; Karrou, M.; Benichou, M. Salt stress effect on epinasty in relation to ethylene production and water relations in tomato. Agronomy 2000, 20, 399–406. [Google Scholar] [CrossRef]
- Zafar, S.A.; Shokat, S.; Ahmed, H.G.M.; Khan, A.; Ali, M.Z.; Atif, R.M. Assessment of salinity tolerance in rice using seedling-based morpho-physiological indices. Adv. Life Sci. 2015, 2, 142–149. [Google Scholar]
- Nievens-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Role and transport of sodium and potassium in plants. Met. Ions Life Sci. 2016, 16, 291–324. [Google Scholar]
- Munns, R.; James, R.A.; Islam, A.K.M.R.; Colmer, T.D. Hordeum marinum-wheat amphiploids maintain higher leaf K+:Na+ and suffer less leaf injury than wheat parents. Plant Soil 2011, 348, 365–377. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Mostofa, M.G.; Akter, M.M.; Srivastava, A.K.; Sayed, M.A.; Hasan, M.S.; Tran, L.P. Impact of salt-induced toxicity on growth and yield-potential of local wheat cultivars: Oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere 2017, 187, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.; Ahmad, I.; Patishtan, J. Regulation of Na(+) fluxes in plants. Front. Plant Sci. 2014, 5, 467. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Martinez, V.; Carvajal, M. Osmotic versus toxic effects of NaCl on pepper plants. Biol. Plant 2008, 52, 72–79. [Google Scholar] [CrossRef]
- Kadri, A.; Madiouni, N. Effet du stress salin sur quelques paramètres biochimiques de la luzerne cultivée (Medicago sativa L.) Mémoire En vue de l’obtention du diplôme de Master Academique. Master’s Thesis, University of Ouargla, Ouargla, Algeria, 4 June 2015. [Google Scholar]
- Martinek, P.; Jirsa, O.; Vaculová, K.; Chrpová, J.; Watanabe, N.; Burešová, V.; Kopecký, D.; Štiasna, K.; Vyhnánek, T.; Trojan, V. Use of wheat gene resources with different grain colour in breeding. In Proceedings of the 64 Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs, Raumber-Gumpenstein, Austria, 25–26 November 2013; pp. 75–78. [Google Scholar]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; John Wiley and Sons, Inc.: New York, NY, USA, 1972; 412p. [Google Scholar]
- Etherton, B. Relationship of cell transmembrane electropotential to potassium and sodium accumulation ratios in oat and pea seedlings. Plant Physiol. 1963, 38, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Cerovic, Z.G.; Pinelli, P.; Tattini, M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 2011, 73, 3–9. [Google Scholar] [CrossRef]
- Troll, W.; Lindsley, J. A photometric method for the determination of proline. J. Biol. Chem. 1955, 215, 655–660. [Google Scholar] [PubMed]
- Wittmer, G. Osmotic and elastic adjustment of durum wheat leaves under drought stress conditions. Genet. Agrar. 1987, 41, 427–436. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts 1. Kinetics and stoichiometry of fatty acid peroxidatoin. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Wheat genotypes | Fresh Weight 1 | Dry Weight 1 | ||||
| Roots | ||||||
| Control | 150 mM | 200 mM | Control | 150 mM | 200 mM | |
| Citrus Yellow | 0.048 ± 0.019 ab | 0.069+0.023 a | 0.23 ± 0.07 b | 0.028 ± 0.009 a | 0.019 ± 0.003 ab | 0.017 ± 0.009 b |
| KM 53-14 Blue | 0.056 ± 0.003 b | 0.042 ± 0.004 a | 0.086 ± 0.02 c | 0.011 ± 0.005 a | 0.015 ± 0.002 a | 0.016 ± 0.008 a |
| KM 178-14 Purple | 0.042 ± 0.001 a | 0.050 ± 0.016 a | 0.13 ± 0.05 b | 0.006 ± 0.001 a | 0.010 ± 0.004 a | 0.013 ± 0.006 a |
| Skorpion Blue Aleur. | 0.091 ± 0.022 a | 0.038 ± 0.014 b | 0.079 ± 0.025 a | 0.007 ± 0.003 a | 0.007 ± 0.001 a | 0.020 ± 0.012 a |
| PS Karkulka | 0.046 ± 0.012 b | 0.039 ± 0.014 b | 0.228 ± 0.091 a | 0.016 ± 0.006 ab | 0.011 ± 0.002 b | 0.023 ± 0.007 a |
| Shoots | ||||||
| Control | 150 mM | 200 mM | Control | 150 mM | 200 mM | |
| Citrus yellow | 0.278 ± 0.076 a | 0.297 ± 0.085 a | 0.233 ± 0.073 a | 0.038 ± 0.011 ab | 0.043 ± 0.014 a | 0.019 ± 0.008 b |
| KM 53-14 Blue | 0.194 ± 0.117 ab | 0.187 ± 0.028 a | 0.086 ± 0.032 b | 0.028 ± 0.014 a | 0.028 ± 0.024 a | 0.018 ± 0.004 a |
| KM 178-14 Purple | 0.252 ± 0.062 a | 0.188 ± 0.06 a | 0.127 ± 0.047 b | 0.032 ± 0.009 a | 0.032 ± 0.012 ab | 0.015 ± 0.007 b |
| Skorpion Blue Aleur. | 0.556 ± 0.085 a | 0.283 ± 0.071 b | 0.079 ± 0.035 c | 0.025 ± 0.009a | 0.042 ± 0.009 a | 0.074 ± 0.013 b |
| PS Karkulka | 0.209 ± 0.069 a | 0.241 ± 0.063 a | 0.228 ± 0.052 a | 0.030 ± 0.011 a | 0.042 ± 0.009 a | 0.046 ± 0.015 a |
| Wheat Cultivars 1 | Salinity Level (mM NaCl) | Water Content (g H2O g−1 DW) | Total Dry Weight (g/plant) | Na+ mmol/g DW | K+ mmol/g DW | K+/Na+ Ratio |
|---|---|---|---|---|---|---|
| Citrus Yellow | 0 mM | 6.30 ± 0.650 b | 0.066 ± 0.016 a | 0.692 ± 0.007 b | 0.179 ± 0.002 b | 0.259 |
| 150 mM | 5.99 ± 0.588 b | 0.062 ± 0.017 a | 1.602 ± 0.097 a | 0.333 ± 0.020 a | 0.208 | |
| 200 mM | 12.15 ± 5.24 a | 0.036 ± 0.017 a | 1.663 ± 0.129 a | 0.345 ± 0.027 a | 0.208 | |
| KM 53-14 Blue | 0 mM | 5.96 ± 1.50 a | 0.039 ± 0.019 a | 0.918 ± 0.085 b | 0.238 ± 0.022 b | 0.259 |
| 150 mM | 6.92 ± 0.341 a | 0.043 ± 0.016 a | 2.582 ± 0.080 a | 0.508 ± 0.016 a | 0.197 | |
| 200 mM | 6.47 ± 5.04 a | 0.034 ± 0.012 a | 2.272 ± 0.162 a | 0.447 ± 0.032 a | 0.197 | |
| KM 178-14 Purple | 0 mM | 6.86 ± 0.91 b | 0.038 ± 0.010 a | 0.643 ± 0.054 c | 0.148 ± 0.012 c | 0.231 |
| 150 mM | 4.65 ± 1.66 b | 0.042 ± 0.011 a | 2.606 ± 0.138 a | 0.455 ± 0.024 a | 0.175 | |
| 200 mM | 9.92 ± 2.79 a | 0.056 ± 0.009 a | 1.597 ± 0.093 b | 0.295 ± 0.017 b | 0.185 | |
| Skorpion Blue Aleurone | 0 mM | 22.11 ± 5.88 a | 0.027 ± 0.012 b | 0.664 ± 0.074 b | 0.153 ± 0.017 b | 0.185 |
| 150 mM | 5.63 ± 0.38 c | 0.049 ± 0.010 b | 1.636 ± 0.093 a | 0.302 ± 0.017 a | 0.185 | |
| 200 mM | 3.98 ± 0.93 b | 0.094 ± 0.025 a | 1.791 ± 0.090 a | 0.331 ± 0.017 a | 0.231 | |
| PS Karkulka | 0 mM | 6.62 ± 1.73 b | 0.046 ± 0.017 a | 0.633 ± 0.023 c | 0.146 ± 0.005 b | 0.231 |
| 150 mM | 4.69 ± 0.46 b | 0.053 ± 0.011 a | 1.634 ± 0.093 b | 0.301 ± 0.017 a | 0.185 | |
| 200 mM | 10.10 ± 3.67 a | 0.069 ± 0.020 a | 1.972 ± 0.063 a | 0.345 ± 0.011 a | 0.175 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbarki, S.; Sytar, O.; Zivcak, M.; Abdelly, C.; Cerda, A.; Brestic, M. Anthocyanins of Coloured Wheat Genotypes in Specific Response to SalStress. Molecules 2018, 23, 1518. https://doi.org/10.3390/molecules23071518
Mbarki S, Sytar O, Zivcak M, Abdelly C, Cerda A, Brestic M. Anthocyanins of Coloured Wheat Genotypes in Specific Response to SalStress. Molecules. 2018; 23(7):1518. https://doi.org/10.3390/molecules23071518
Chicago/Turabian StyleMbarki, Sonia, Oksana Sytar, Marek Zivcak, Chedly Abdelly, Artemio Cerda, and Marian Brestic. 2018. "Anthocyanins of Coloured Wheat Genotypes in Specific Response to SalStress" Molecules 23, no. 7: 1518. https://doi.org/10.3390/molecules23071518
APA StyleMbarki, S., Sytar, O., Zivcak, M., Abdelly, C., Cerda, A., & Brestic, M. (2018). Anthocyanins of Coloured Wheat Genotypes in Specific Response to SalStress. Molecules, 23(7), 1518. https://doi.org/10.3390/molecules23071518