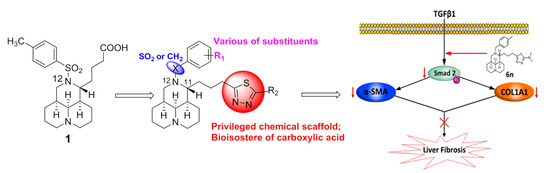
3.2.2. General Procedure for the Synthesis of Compounds 6a–o
A mixture of 1 or 5a–d (2.5 mmol), thiosemicarbazide or N-substituted thiosemicarbazide (2.5 mmol), methanesulfonic acid (12.5 mmol) and phosphorus pentoxide (2.5 mmol) were heated at 70 °C for 8 h. The reaction mixture was poured into ice water (40 mL) and neutralized with aqueous ammonia to pH 8. The solution was extracted with dichloromethane (50 mL), and the organic layer was washed by saturated aqueous sodium chloride (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel with dichloromethane/methanol or petroleum ether/ethyl acetate as the eluent.
12-N-p-Methlybenzenesulfonyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6a). Compound 1 (1.04 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluent to give the desired product 6a as a white solid, yield: 40.9%; m.p.: 237 °C (dec.); = −6.16; 1H-NMR (500 MHz, DMSO-d6) δ 7.68 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.38 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 7.00 (s, 2H, 9′-NH2), 3.42–3.36 (m, 2H, 3′-CH2), 3.16–3.08 (m, 1H, 6-CH), 2.73–2.69 (m, 1H, 5-CH), 2.65–2.61 (m, 1H, 7-CH), 2.59–2.56 (m, 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.95–1.90 (m, 1H, 1′a-CH), 1.87–1.80 (m, 2H, 2′-CH2), 1.76–1.61 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.51–1.45 (m, 2H, 4-CH2), 1.39–1.23 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, DMSO-d6) δ 168.2 (7′-C), 158.2 (4′-C), 142.9 (18-C), 136.7 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.2 (2-CH2), 56.1 (10-CH2), 47.1 (13-CH2), 38.6 (7-CH), 34.0 (5-CH), 29.7 (1′-CH2), 29.6 (3′-CH2), 27.6 (4-CH2), 27.5 (8-CH2), 24.8 (2′-CH2), 21.1 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C23H34O2N5S2 [M + H]+, 476.2148; Found, 476.2149; HPLC: tR = 10.29 min, normalization method purity 97.11%.
12-N-p-Mmethlybenzenesulfonyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6b). Compound 1 (1.04 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give to give the desired product 6b as a white solid, yield: 33.4%; m.p.: 169–170 °C; = −7.98; 1H-NMR (500 MHz, DMSO-d6) δ 7.68 (d, J = 7.9 Hz, 2H, 16-CH, 20-CH), 7.49 (q, J = 4.8 Hz, 1H, 9′-NH), 7.38 (d, J = 7.9 Hz, 2H, 17-CH, 19-CH), 3.43–3.35 (m, 2H, 3′-CH2), 3.14–3.09 (m, 1H, 6-CH), 2.83 (d, J = 4.8 Hz, 3H, 9′-NCH3), 2.76–2.70 (m, 1H, 5-CH), 2.67–2.61 (m, 1H, 7-CH), 2.57 (m 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.95–1.90 (m, 1H, 1′a-CH), 1.86–1.80 (m, 2H, 2′-CH2), 1.75–1.62 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.49–1.23 (m, 9H, 3-CH2, 4-CH2, 8-CH2, 9-CH2, 1′b-CH ); 13C-NMR (126 MHz, DMSO-d6) δ 169.1 (7′-C), 157.6 (4′-C), 142.9 (18-C), 136.7 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 47.0 (13-CH2), 38.6 (7-CH), 34.0 (5-CH), 31.2 (9′-NCH3), 29.7 (1′-CH2), 29.6 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 24.9 (2′-CH2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C24H36O2N5S2 [M + H]+, 490.2304; Found, 490.2304; HPLC: tR = 10.78 min, normalization method purity 98.74%.
12-N-p-Methylbenzenesulfonyl-3′-(N-iso-propyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6c). Compound 1 (1.04 g, 2.5 mmol) was treated with 4-iso-propyl-3-thiosemicarbazide (0.33 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give the desired product 6d as a white solid, yield: 34.1%; m.p.: 142–143 °C; = −15.79; 1H-NMR (600 MHz, DMSO-d6) δ 7.68 (d, J = 8.1 Hz, 2H, 16-CH, 20-CH), 7.44 (d, J = 7.2 Hz, 1H, 9′-NH), 7.38 (d, J = 8.1 Hz, 2H, 17-CH, 19-CH), 3.77–3.69 (m, 1H, 9′-NCH), 3.42–3.38 (m, 2H, 3′-CH2), 3.13 (t, J = 11.6 Hz, 1H, 6-CH), 2.74–2.70 (m, 1H, 5-CH), 2.66–2.61 (m, 1H, 7-CH), 2.58–2.53 (m, 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.94 (m, 1H, 1′a-CH), 1.86–1.80 (m, 2H, 2′-CH2), 1.75–1.61 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.49–1.46 (m, 2H, 4-CH2), 1.39–1.23 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH), 1.17 (d, J = 6.4 Hz, 6H, NC(CH3)2); 13C-NMR (151 MHz, DMSO-d6) δ 167.4 (7′-C), 157.2 (4′-C), 142.9 (18-C), 136.7 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 47.0 (13-CH2), 46.5 (NCH), 38.6 (7-CH), 34.0 (5-CH), 29.8 (1′-CH2), 29.5 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 24.9 (2′-CH2), 22.2 (NC(CH3)2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C26H40O2N5S2 [M + H]+, 518.2617; Found, 518.2607; HPLC: tR = 12.11 min, normalization method purity 97.99%.
12-N-p-Methylbenzenesulfonyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6d). Compound 1 (1.04 g, 2.5 mmol) was treated with 4,4-dimethyl-3-thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give the desired product 6e as a white solid, yield: 24.2%; m.p.: 171–172 °C; = −9.66; 1H-NMR (500 MHz, DMSO-d6) δ 7.68 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.39 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 3.45–3.36 (m, 2H, 3′-CH2), 3.18–3.07 (m, 1H, 6-CH), 3.03 (s, 6H, N(CH3)2), 2.81–2.72 (m, 1H, 5-CH), 2.72–2.63 (m, 1H, 7-CH), 2.57 (d, J = 11.4 Hz, 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.94 (m, 1H, 1′a-CH), 1.90–1.79 (m, 2H, 2′-CH2), 1.78–1.60 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.54–1.18 (m, 9H, 3-CH2, 4-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, DMSO-d6) δ 171.2 (7′-C), 158.5 (4′-C), 142.8 (18-C), 136.8 (15-C), 129.5 (17-CH, 19-CH), 127.1 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 47.0 (13-CH2), 41.1 (N(CH3)2), 38.7 (7-CH), 34.0 (5-CH), 29.7 (1′-CH2), 29.6 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 25.1 (2′-CH2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C25H38O2N5S2 [M + H]+, 504.2441; Found, 504.2447; HPLC: tR = 11.59 min, normalization method purity 98.45%.
12-N-p-Methylbenzenesulfonyl-3′-(N-phenyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6e). Compound 1 (1.04 g, 2.5 mmol) was treated with 4-phenyl-3-thiosemicarbazide (0.42 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate as the eluents to give the desired product 6c as a white solid, yield: 48.2%; m.p.: 193–194 °C; = −13.89; 1H-NMR (500 MHz, DMSO-d6) δ 10.26 (s, 1H, 9′-NH), 7.68 (d, J = 8.0 Hz, 2H, 16-CH, 20-CH), 7.60 (d, J = 8.0 Hz, 2H, 17-CH, 19-CH), 7.38 (d, J = 7.5 Hz, 2H, 2 × 9′-NCHarom), 7.33 (t, J = 7.5 Hz, 2H, 2 × 9′-NCHarom), 6.97 (t, J = 7.5 Hz, 1H, 9′-NCHarom ), 3.43–3.38 (m, 2H, 3′-CH2), 3.13 (t, J = 11.6 Hz, 1H, 6-CH), 2.87–2.81 (m, 1H, 5-CH), 2.79–2.72 (m, 1H, 7-CH), 2.57 (d, J = 11.1 Hz, 1H, 11-CH), 2.35 (s, 3H, Ph-CH3), 1.93 (m, 1H, 1′a-CH), 1.86–1.83 (m, 2H, 2′-CH2), 1.75–1.68 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.56–1.23 (m, 9H, 3-CH2, 4-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, DMSO-d6) δ 163.9 (7′-C), 159.5 (4′-C), 142.8 (18-C), 140.8 (9′N-Ph), 136.8 (15-C), 129.5 (17-CH, 19-CH), 129.0 (9′N-Ph), 127.1 (16-CH, 20-CH), 121.6 (9′N-Ph), 117.1 (9′N-Ph), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 46.9 (13-CH2), 38.8 (7-CH), 34.0 (5-CH), 29.8 (1′-CH2), 29.4 (3′-CH2), 27.6 (4-CH2), 27.5 (8-CH2), 24.9 (2′-CH2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C29H38O2N5S2 [M + H]+, 552.2461; Found, 552.2462; HPLC: tR = 13.39 min, normalization method purity 96.69%.
12-N-p-Ttrifluoromethlybenzenesulfonyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6f). Compound 5a (1.19 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give the desired product 6f as a white solid, yield: 48.0%; m.p.: 206 °C (dec.); = −0.65; 1H-NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.73 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 5.56 (s, 2H, 9′-NH2), 3.67–3.57 (m, 1H, 3′a-CH), 3.53–3.45 (m, 1H, 3′b-CH), 3.22 (t, J = 11.5 Hz, 1H, 6-CH), 2.93–2.75 (m, 2H, 5-CH, 7-CH), 2.54 (d, J = 11.5 Hz, 1H, 13a-CH), 2.45 (d, J = 11.5 Hz, 1H, 13b-CH), 1.96 (m, 2H, 2′-CH2), 1.89–1.62 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.49–1.26 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 168.2 (7′-C), 161.2 (4′-C), 143.9 (15-C), 133.9 (18-C), 128.1 (17-CH, 19-CH), 125.9 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.7 (6-CH), 57.5 (11-CH), 56.6 (2-CH2), 56.5 (10-CH2), 46.9 (13-CH2), 39.7 (7-CH), 34.4 (5-CH), 31.6 (1′-CH2), 30.3 (3′-CH2), 28.3 (4-CH2), 27.9 (8-CH2), 25.8 (2′-CH2), 20.9 (3-CH2), 20.6 (9-CH2); ESI-HRMS: m/z Calcd for C23H31O2N5F3S2 [M + H]+, 530.1865; found, 530.1864; HPLC: tR = 11.41 min, normalization method purity 98.85%.
12-N-p-Trifluoromethlybenzenesulfonyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6g). Compound 5a (1.19 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6g as a white solid, yield: 45.0%; m.p.: 181 °C (dec.); = −1.89; 1H-NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.73 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 5.79 (s, 1H, 9′-NH), 3.68–3.57 (m, 1H, 3′a-CH ), 3.53–3.46 (m, 1H, 3′b-CH), 3.24 (t, J = 11.4 Hz, 1H, 6-CH), 3.01 (s, 3H, 9′-NCH3), 2.91–2.77 (m, 2H, 5-CH, 7-CH), 2.54 (d, J = 11.4 Hz, 1H, 13a-CH), 2.46 (d, J = 11.4 Hz, 1H, 13b-CH), 2.02–1.91 (m, 2H, 2′-CH2), 1.86–1.63 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.51–1.31 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 171.4 (7′-C), 159.2 (4′-C), 144.1 (15-C), 133.9 (18-C), 128.1 (17-CH, 19-CH), 125.8 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.7 (6-CH), 57.6 (11-CH), 56.6 (2-CH2), 56.5 (10-CH2), 47.0 (13-CH2), 39.8 (7-CH), 34.5 (5-CH), 33.3 (9′-NCH3), 31.6 (1′-CH2), 30.4 (3′-CH2), 28.3 (4-CH2), 28.0 (8-CH2), 25.9 (2′-CH2), 20.9 (3-CH2), 20.7 (9-CH2); ESI-HRMS: m/z Calcd for C24H33O2N5F3S2 [M + H]+, 544.2022; found, 544.2019; HPLC: tR = 11.89 min, normalization method purity 98.90%.
12-N-p-Trifluoromethlybenzenesulfonyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6h). Compound 5a (1.19 g, 2.5 mmol) was treated with 4,4-dimethyl-3- thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate to give the desired product 6h as a white solid, yield: 33.3%; m.p.: 147–148 °C; = −3.30; 1H-NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.72 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 3.66–3.62 (m, 1H, 3′a-CH), 3.51 (dd, J = 12.6, 6.2 Hz, 1H, 3′b-CH), 3.27–3.22 (m, 1H, 6-CH), 3.09 (s, 6H, 9′-N(CH3)2), 2.90–2.71 (m, 2H, 5-CH, 7-CH), 2.56–2.53 (m, 1H, 13a-CH), 2.49–2.45 (m, 1H, 13b-CH), 1.99–1.94 (m, 2H, 2′-CH2), 1.86–1.61 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.52–1.29 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 172.0 (7′-C), 159.3 (4′-C), 144.2 (15-C), 133.8 (18-C), 128.1 (17-CH, 19-CH), 125.8 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.8 (6-CH), 57.6 (11-CH), 56.6 (2-CH2), 56.5 (10-CH2), 47.1 (13-CH2), 41.5 (9′N(CH3)2), 39.8 (7-CH), 34.5 (5-CH), 31.5 (1′-CH2), 30.4 (3′-CH2), 28.3 (4-CH2), 28.0 (8-CH2), 25.9 (2′-CH2), 20.9 (3-CH2), 20.7 (9-CH2); ESI-HRMS: m/z Calcd for C25H35O2N5F3S2 [M + H]+, 558.2178; found, 558.2169; HPLC: tR = 12.71 min, normalization method purity 98.60%.
12-N-Benzyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6i) Compound 5b (1.19 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6i as a white solid, yield: 34.7%; m.p.: 170–171 °C; = −29.79; 1H-NMR (600 MHz, CDCl3) δ 7.30 (dt, J = 15.0, 7.2 Hz, 4H, 16-CH, 17-CH, 19-CH, 20-CH), 7.20 (t, J = 7.2 Hz, 1H, 18-CH), 5.67 (s, 2H, 9′-NH2), 4.00 (d, J = 13.4 Hz, 1H, 14a-CH), 3.12 (d, J = 13.4 Hz, 1H, 14b-CH), 2.94–2.72 (m, 5H, 3′-CH2, 5-CH, 7-CH, 11-CH ), 2.64 (t, J = 11.9 Hz, 1H, 6-CH), 2.33 (m, 1H, 1′a-CH), 1.95–1.57 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.45–1.28 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 168.3 (7′-C), 161.6 (4′-C), 140.6 (15-C), 128.7 (17-CH, 19-CH), 128.3 (16-CH, 20-CH), 126.7 (18-CH), 64.6 (6-CH), 57.6 (11-CH), 57.4 (14-CH2), 57.3 (13-CH2), 56.7 (2-CH2), 52.4 (10-CH2), 37.9 (7-CH), 34.0 (5-CH), 30.8 (1′-CH2), 28.3 (3′-CH2), 28.2 (4-CH2), 27.4 (8-CH2), 23.9 (2′-CH2), 21.7 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C23H34N5S [M + H]+, 412.2529; found, 412.2530; HPLC: tR = 8.26 min, normalization method purity 98.65%.
12-N-Benzyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6j) Compound 5b (1.19 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6j as a white solid, yield: 32.3%; m.p.: 137–138 °C; = −28.80; 1H-NMR (600 MHz, CDCl3) δ 7.32–7.27 (m, 4H, 16-CH, 17-CH, 19-CH, 20-CH), 7.20 (t, J = 7.2 Hz, 1H, 18-CH), 5.86 (m, 1H, 9′-NH), 4.01 (d, J = 13.0 Hz, 1H, 14a-CH), 3.12 (d, J = 13.0 Hz, 1H, 14b-CH), 2.97–2.60 (m, 9H, 3′-CH2, 5-CH, 6-CH, 7-CH, 9′-NCH3, 11-CH), 2.34 (d, J = 11.6 Hz, 1H, 1′a-CH), 1.99–1.52 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.47–1.29 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 171.4 (7′-C), 159.7 (4′-C), 140.6 (15-C), 128.7 (17-CH, 19-CH), 128.3 (16-CH, 20-CH), 126.7 (18-CH), 64.6 (6-CH), 57.7 (11-CH), 57.4 (14-CH2), 57.3 (13-CH2), 56.7 (2-CH2), 52.3 (10-CH2), 37.9 (7-CH), 34.0 (5-CH), 33.3 (9′-NCH3), 30.8 (1′-CH2), 28.3 (3′-CH2), 28.2 (4-CH2), 27.4 (8-CH2), 24.0 (2′-CH2), 21.7 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C24H36N5S [M + H]+, 426.2686; found, 426.2685; HPLC: tR = 9.14 min, normalization method purity 98.91%.
12-N-Benzyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane hydrochloride (6k). Compound 5b (1.19 g, 2.5 mmol) was treated with 4,4-dimethyl-3-thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate to give the crude product and treated with 2 N hydrochloride/ether (3 mL) to give the desired product 6k as a white solid, yield: 16.3%; m.p.: 189–190 °C; = −14.64; 1H-NMR (500 MHz, DMSO-d6) δ 11.81 (s, 1H, 1-NH+), 7.66–7.62 (m, 2H, 16-CH, 20-CH), 7.47–7.43 (m, 3H, 17-CH, 18-CH, 19-CH), 5.00 (d, J = 12.7 Hz, 2H, 14-CH2), 4.26 (t, J = 10.3 Hz, 1H, 6-CH), 3.99–3.93 (m, 2H, 13-CH2), 3.61 (d, J = 10.3 Hz, 1H, 7-CH), 3.32–3.20 (m, 2H, 3′-CH2), 3.05–2.87 (m, 6H, 9′-N(CH3)2), 2.66–2.60 (m, 2H, 5-CH, 11-CH), 2.57–2.52 (m, 1H, 1′a-CH), 2.18–1.92 (m, 6H, 2-CH2, 10-CH2, 2′-CH2), 1.89–1.55 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2), 1.43 (d, J = 13.6 Hz, 1H, 1′b-CH); 13C-NMR (126 MHz, DMSO-d6) δ 171.0 (7′-C), 158.7 (4′-C), 132.0 (18-C), 130.7 (15-C), 123.0 (17-CH, 19-CH), 129.4 (16-CH, 20-CH), 61.0 (6-CH), 60.8 (14-CH2), 58.1 (11-CH), 54.8 (2-CH2), 54.7 (10-CH2), 49.3 (13-CH2), 43.0 (9′-N(CH3)2), 36.5 (7-CH), 30.5 (5-CH), 29.9 (1′-CH2), 28.2 (3′-CH2), 26.4 (4-CH2), 24.7 (8-CH2), 24.2 (2′-CH2), 18.5 (3-CH2), 18.4 (9-CH2); ESI-HRMS: m/z Calcd for C25H38N5S [M-HCl+H]+, 440.2842; found, 440.2839; HPLC: tR = 9.40 min, normalization method purity 98.49%.
12-N-p-Methylbenzyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6l) Compound 5c (0.93 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6l as a white solid, yield: 41.4%; m.p.:167–168 °C; = −30.62; 1H-NMR (600 MHz, CDCl3) δ 7.19 (d, J = 7.7 Hz, 2H, 16-CH, 20-CH), 7.09 (d, J = 7.7 Hz, 2H, 17-CH, 19-CH), 5.68 (s, 2H, 9′-NH2), 3.96 (d, J = 13.4 Hz, 1H, 14a-CH), 3.08 (d, J = 13.4 Hz, 1H, 14b-CH), 2.89–2.73 (m, 4H, 3′-CH2, 5-CH, 7-CH), 2.62 (t, J = 11.9 Hz, 1H, 6-CH), 2.35–2.32 (m, 1H, 11-CH), 2.31 (s, 3H, Ph-CH3), 2.04–2.00 (m, 1H, 1′a-CH), 1.92–1.60 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2 ), 1.43–1.29 (m, 5H, 3-CH2, 9-CH2, 1′b-CH ); 13C-NMR (151 MHz, CDCl3) δ 168.3 (7′-C), 161.6 (4′-C), 137.3 (18-C), 136.2 (15-C), 129.0 (17-CH, 19-CH), 128.7 (16-CH, 20-CH), 64.6 (6-CH), 57.6 (14-CH2), 57.4 (11-CH), 56.4 (2-CH2), 52.2 (10-CH2), 37.9 (13-CH2), 33.9 (7-CH), 30.8 (5-CH), 28.3 (1′-CH2), 28.2 (3′-CH2), 27.4 (4-CH2), 23.9 (8-CH2), 21.7 (2′-CH2), 21.4 (3-CH2), 21.2 (9-CH2), 21.1 (Ph-CH3); ESI-HRMS: m/z Calcd for C24H36N5S [M + H]+, 426.2686; found; 426.2685; HPLC: tR =8.78 min, normalization method purity 97.53%.
12-N-p-Methylbenzyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6m). Compound 5c (0.93 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6m as a white solid, yield: 34.1%; m.p.: 163–164 °C; = −35.69; 1H-NMR (600 MHz, CDCl3) δ 7.19 (d, J = 7.6 Hz, 2H, 16-CH, 20-CH), 7.08 (d, J = 7.6 Hz, 2H, 17-CH, 19-CH), 6.28 (s, 1H, 9′-NH), 3.96 (d, J = 13.3 Hz, 1H, 14a-CH), 3.06 (d, J = 13.3 Hz, 1H, 14b-CH), 2.94 (s, 3H, 9′-NCH3), 2.89–2.77 (m, 4H, 3′-CH2, 5-CH, 7-CH), 2.61 (t, J = 12.0 Hz, 1H, 6-CH), 2.35–2.32 (m, 1H, 11-CH), 2.30 (s, 3H, Ph-CH3), 2.01 (m, 1H, 1′a-CH), 1.95–1.55 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.44–1.29 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 171.6 (7′-C), 159.5 (4′-C), 137.4 (18-C), 136.1 (15-C), 128.9 (17-CH, 19-CH), 128.6 (16-CH, 20-CH), 64.6 (6-CH), 57.7 (14-CH2), 57.4 (11-CH), 56.4 (2-CH2), 52.2 (10-CH2), 38.0 (13-CH2), 34.0 (7-CH), 33.3 (9′-NCH3), 30.8 (5-CH), 28.3 (1′-CH2), 28.2 (3′-CH2), 27.4 (4-CH2), 24.0 (8-CH2), 21.7 (2′-CH2), 21.4 (3-CH2), 21.2 (9-CH2), 21.1 (Ph-CH3); ESI-HRMS: m/z Calcd for C25H38N5S [M + H]+, 440.2842; found, 440.2845; HPLC: tR =10.09 min, normalization method purity 95.47%.
12-N-p-Methylbenzyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane hydrochloride (6n). Compound 5c (0.93 g, 2.5 mmol) was treated with 4,4-dimethyl-3-thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate to give the crude product and treated with 2 N hydrochloride/ether (3 mL) to give the desired product 6n as a white solid, yield: 21.1%; m.p.: 199–200 °C; = −17.19; 1H-NMR (500 MHz, DMSO-d6) δ 7.51 (d, J = 7.6 Hz, 2H, 16-CH, 20-CH), 7.25 (d, J = 7.6 Hz, 2H, 17-CH, 19-CH), 5.76 (s, 1H, 12-NH), 4.94 (m, 1H, 5-CH), 4.24 (m, 1H, 6-CH), 3.98–3.84 (m, 2H, 14-CH2), 3.62 (m, 1H, 11-CH), 3.24 (m, 2H, 13-CH2), 3.17 (s, 6H, 9′-N(CH3)2), 3.05–2.86 (m, 4H, 2-CH2,10-CH2 ), 2.62 (d, J = 10.3 Hz, 2H, 3′-CH2), 2.32 (s, 3H, Ph-CH3), 2.17–2.07 (m, 1H, 7-CH), 1.98 (m, 3H, 1′a-CH, 2′-CH2), 1.87–1.55 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2), 1.43 (d, J = 13.8 Hz, 1H, 1′b-CH).; 13C-NMR (126 MHz, DMSO-d6) δ 170.5 (7′-C), 158.0 (4′-C), 138.8 (18-C), 131.4 (15-C), 129.4 (17-CH, 19-CH), 126.9 (16-CH, 20-CH), 60.3 (6-CH), 60.2 (14-CH2), 57.2 (11-CH), 55.0 (2-CH2), 54.2 (10-CH2), 48.5 (13-CH2), 42.3 (N(CH3)2), 35.9 (7-CH), 29.9 (5-CH), 29.2 (1′-CH2), 27.6 (3′-CH2), 25.8 (4-CH2), 24.1 (8-CH2), 23.5 (2′-CH2), 20.9 (3-CH2), 17.9 (9-CH2), 17.8 (Ph-CH3); ESI-HRMS: m/z Calcd for C26H40N5S [M − HCl + H]+, 454.2999 found, 454.2996; HPLC: tR = 9.84 min, normalization method purity 96.00%.
12-N-p-Bromobenzyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6o) Compound 5d (1.09 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6o as a white solid, yield: 44.1%; m.p.: 193–194 °C; = −19.58; 1H-NMR (500 MHz, CDCl3) δ 7.39 (d, J = 8.0 Hz, 2H, 16-CH, 20-CH), 7.19 (d, J = 8.0 Hz, 2H, 17-CH, 19-CH), 5.61 (s, 2H, 9′-NH2), 3.92 (d, J = 13.8 Hz, 1H, 14a-CH), 3.06 (d, J = 13.8 Hz, 1H,14b-CH), 2.91–2.75 (m, 5H, 3′-CH2, 5-CH, 7-CH, 11-CH), 2.64 (t, J = 11.8 Hz, 1H, 6-CH), 2.27 (m, 1H, 1′a-CH), 1.94–1.55 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.45–1.30 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 168.2 (7′-C), 161.5 (4′-C), 139.6 (15-C), 131.4 (17-CH, 19-CH), 130.3 (16-CH, 20-CH), 120.3 (18-C), 64.5 (6-CH), 57.6 (11-CH), 57.4 (14-CH2), 57.2 (13-CH2), 55.9 (2-CH2), 52.3(10-CH2), 37.9 (7-CH), 33.9 (5-CH), 30.7(1′-CH2), 28.2 (3′-CH2), 28.1 (4-CH2), 27.4 (8-CH2), 23.8 (2′-CH2), 21.6 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C23H33N5BrS [M + H]+, 490.1635; found, 490.1635; HPLC: tR = 9.88 min; normalization method purity 97.24%.
3.2.3. General Procedure for the Synthesis of Compounds 7a–d
To a solution of 6a, 6f, 6i or 6l (0.40 mmol) in dichloromethane (10 mL), triethylamine (0.25 g, 2.5 mmol) and acetyl chloride (0.16 g, 2 mmol) were added at 0 °C and stirred at room temperature, until the TLC analysis showed completion of the reaction. The reaction mixture was washed by saturated aqueous ammonium chloride (10 mL × 2) and saturated aqueous sodium chloride (10 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel with dichloromethane/methanol as the eluents to give 7a–d.
12-N-p-Methylblenzenesulfonyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7a). White solid, yield: 93.7%; m.p.: 273 °C (dec,); = −4.83; 1H-NMR (500 MHz, DMSO-d6) δ 12.39 (s, 1H, 9′-NH), 7.68 (d, J = 8.0 Hz, 2H, 16-CH, 20-CH), 7.37 (d, J = 8.0 Hz, 2H, 17-CH, 19-CH), 3.46–3.38 (m, 2H, 3′-CH2), 3.14 (t, J = 11.7 Hz, 1H, 6-CH), 2.92–2.84 (m, 1H, 5-CH), 2.83–2.74 (m, 1H, 7-CH), 2.62–2.51 (m, 1H, 11-CH), 2.37 (s, 3H, Ph-CH3), 2.17 (s, 3H, 11′CH3), 1.96–1.92 (m, 1H, 1a’-CH), 1.89–1.81 (m, 2H, 2′-CH2), 1.77–1.66 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 13-CH2), 1.58–1.22 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, DMSO-d6) δ 168.4 (7′-C), 163.7 (10′-CO), 158.1 (4′-C), 142.9 (18-C), 136.8 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.4 (6-CH), 56.7 (11-CH), 56.2 (2-CH2), 56.1 (10-CH2), 47.2 (13-CH2), 38.7 (7-CH), 34.2 (5-CH), 29.6 (1′-CH2), 29.0 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 25.1(2′-CH2), 22.4 (11′-CH3), 21.0 (Ph-CH3), 20.4 (3-CH2), 20.3 (9-CH2); ESI-HRMS: m/z Calcd for C25H36O3N5S2 [M + H]+, 518.2254; found, 518.2252; HPLC: tR = 11.66 min, normalization method purity 95.35%.
12-N-p-Trifluoromethlybenzenesulfonyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7b). White solid, yield: 99.1%; m.p.: 197 °C (dec,); = −1.30; 1H-NMR (600 MHz, CDCl3) δ 13.27 (s, 1H, 9′-NH), 7.98 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.73 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 3.66–3.57 (m, 1H, 3′a-CH), 3.53–3.42 (m, 1H, 3′b-CH), 3.25 (t, J = 11.6 Hz, 1H, 6-CH), 3.03–2.91 (m, 2H, 5-CH, 7-CH), 2.54 (d, J = 11.4 Hz, 1H, 13a-CH), 2.49–2.42 (m, 4H, 13b-CH, 11′-CH3), 1.99–1.96 (m, 2H, 2′-CH2), 1.88–1.74 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.52–1.29 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 168.9 (7′-C), 164.6 (10′-CO), 160.5 (4′-C), 144.1 (15-C), 134.0 (18-C), 128.1 (17-CH, 19-CH), 125.8 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.7 (6-CH), 57.5 (11-CH), 56.7 (2-CH2), 56.6 (10-CH2), 46.9 (13-CH2), 39.8 (7-CH), 34.4 (5-CH), 31.8 (1′-CH2), 29.8 (3′-CH2), 28.4 (4-CH2), 28.0 (8-CH2), 25.8 (2′-CH2), 23.3 (11′-CH3), 20.9 (3-CH2), 20.7 (9-CH2); ESI-HRMS: m/z Calcd for C25H33O3N5F3S2 [M + H]+, 572.1971; found, 572.1970; HPLC: tR =11.91 min, normalization method purity 99.53%.
12-N-Benzyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7c). White solid, yield: 93.5%; m.p.: 188–189 °C; = −27.65; 1H-NMR (500 MHz, CDCl3) δ 13.31 (s, 1H, 9′-NH), δ 7.32–7.27 (m, 4H, 16-CH, 17-CH, 19-CH, 20-CH), 7.20 (t, J = 7.2 Hz, 1H, 18-CH), 3.98 (d, J = 13.4 Hz, 1H, 14a-CH), 3.10 (d, J = 13.4 Hz, 1H, 14b-CH), 2.97–2.73 (m, 5H, 3′-CH2, 5-CH, 7-CH, 11-CH), 2.63 (t, J = 12.0 Hz, 1H, 6-CH), 2.42 (s, 3H, 11′-CH3), 2.37–2.31 (m, 4H, 2-CH2,10-CH2), 2.06–1.78 (m, 8H, 4-CH2, 8-CH2, 13-CH2, 2′-CH2), 1.47–1.22 (m, 6H, 3-CH2, 9-CH2, 1′-CH2); 13C-NMR (126 MHz, CDCl3) δ 169.0 (7′-C), 165.0 (10′-CO), 160.5 (4′-C), 140.4 (15-C), 128.6 (17-CH, 19-CH), 128.4 (16-CH, 20-CH), 126.8 (18-CH), 64.6 (6-CH), 57.6 (11-CH), 57.4 (14-CH2), 56.9 (13-CH2), 56.7 (2-CH2), 52.4 (10-CH2), 37.9 (7-CH), 34.0 (5-CH), 30.2 (1′-CH2), 28.4 (3′-CH2), 28.2 (4-CH2), 27.4 (8-CH2), 23.9 (11′-CH3), 23.3 (2′-CH2), 21.6 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C25H36ON5S [M + H]+, 454.2662; found, 454.2662; HPLC: tR = 8.96 min, normalization method purity 98.36%.
12-N-p-Methylbenzyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7d). White solid, yield: 91.5%; m.p.: 226 °C (dec.); = −31.74; 1H-NMR (500 MHz, CDCl3) δ 13.34 (s, 1H, 9′-NH), 7.19 (d, J = 7.6 Hz, 2H, 16-CH, 20-CH), 7.09 (d, J = 7.6 Hz, 2H, 17-CH, 19-CH), 3.98 (d, J = 13.3 Hz, 1H, 14a-CH), 3.10 (d, J = 13.3 Hz, 1H, 14b-CH), 2.95–2.86 (m, 2H, 3′-CH2), 2.83 (m, 1H, 5-CH), 2.76 (m, 1H, 7-CH), 2.63 (t, J = 12.0 Hz, 1H, 6-CH), 2.42 (s, 3H, 11′-CH3), 2.36 (d, J = 11.0 Hz, 1H, 11-CH), 2.31 (s, 3H, Ph-CH3), 2.03 (s, 1H, 1′a-CH), 1.92–1.78 (m, 6H, 2-CH2, 4-CH2, 8-CH2,), 1.75–1.62 (m, 4H, 10-CH2, 2′-CH2 ), 1.48–1.30 (m, 6H,3-CH2, 9-CH2, 13-CH2), 1.25 (s, 1H, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 169.0 (7′-C), 165.1 (10′-CO), 160.5 (4′-C), 137.4 (18-C), 136.2 (15-C), 129.0 (17-CH, 19-CH), 128.6 (16-CH, 20-CH), 64.6 (6-CH), 57.7 (14-CH2), 57.5 (11-CH), 56.7 (2-CH2), 52.4 (10-CH2), 38.0 (13-CH2), 34.1 (7-CH), 30.2 (5-CH), 29.8 11′-CH3), 28.5 (1′-CH2), 28.2 (3′-CH2), 27.4 (4-CH2), 24.0 (8-CH2), 23.3 (2′-CH2), 21.7 (3-CH2), 21.5 (9-CH2), 21.2 (Ph-CH3); ESI-HRMS: m/z Calcd for C26H38ON5S [M + H]+, 468.2792; found, 468.2789; HPLC: tR =9.34 min, normalization method purity 98.77%.