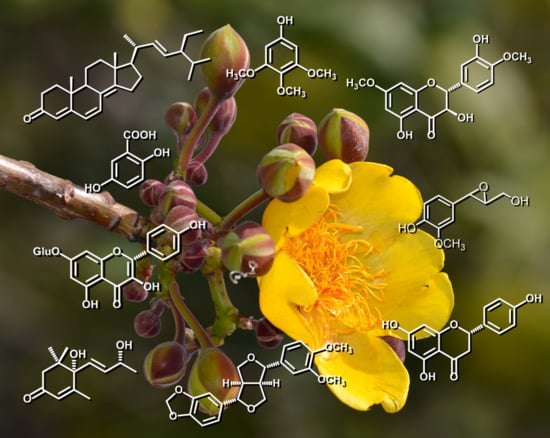
Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamien-Meda, A.; Kiendrebeogo, M.; Compaoré, M.; Meda, R.N.T.; Bacher, M.; Koenig, K.; Pacher, T.; Fuehrer, H.-P.; Noedl, H.; Willcox, M.; et al. Quality assessment and antiplasmodial activity of West African Cochlospermum species. Phytochemistry 2015, 119, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.F.; Knox, J.R. Flavonoids from Cochlospermum gillivraei. Phytochemistry 1975, 14, 2510–2511. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Suresh, K.I.; Rama Rao, B.; Vijaya Saradhi, U.V.R.; Prabhakar Rao, T. Morphological, physico-chemical and structural characterization of gum kondagogu (Cochlospermum gossypium): A tree gum from India. Food Hydrocoll. 2008, 22, 899–915. [Google Scholar] [CrossRef]
- Hongsing, P.; Palanuvej, C.; Ruangrungsi, N. Chemical compositions and biological activities of selected exudate gums. J. Chem. Pharm. Res. 2012, 4, 4174–4180. [Google Scholar]
- Bate-Smith, E.C. Chemotaxonomie der Pflanzen. Bull. Soc. Bot. Mem. 1964, 435. [Google Scholar] [CrossRef]
- Anaga, A.O.; Oparah, N. Investigation of the methanol root extract of Cochlospermum planchonii for pharmacological activities in vitro and in vivo. Pharm. Biol. 2009, 47, 1027–1034. [Google Scholar] [CrossRef]
- Solon, S.; Carollo, C.A.; Brandão, L.F.G.; Macedo, C.; Klein, A.; Dias-Junior, C.A.; Siqueira, J.M. Phenolic derivatives and other chemical compounds from Cochlospermum regium. Quim. Nova 2012, 35, 1169–1172. [Google Scholar] [CrossRef]
- Diallo, B.; Vanhaelen, M.; Vanhaelen-Fastré, R.; Konoshima, T.; Kozuka, M.; Tokuda, H. Studies on inhibitors of skin-tumor promotion. Inhibitory effects of triterpenes from Cochlospermum tinctorium on Epstein-Barr Virus Activation. J. Nat. Prod. 1989, 52, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Diallo, B.; Vanhaelen-Fastré, R.; Vanhaelen, M. Triacylbenzenes and long-chain volatile ketones from Cochlospermum tinctorium rhizome. Phytochemistry 1991, 30, 4153–4156. [Google Scholar] [CrossRef]
- Ballin, N.Z.; Traore, M.; Tinto, H.; Sittie, A.; Mølgaard, P.; Olsen, C.E.; Kharazmi, A.; Christensen, S.B. Antiplasmodial compounds from Cochlospermum tinctorium. J. Nat. Prod. 2002, 65, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Xenofonte de Almeida, S.C.; Gomes de Lemos, L.T.; Rocha Silveira, E.; Loiola Pessoa, O.D. Constituintes químicos voláteis e não-voláteis de Cochlospermum vitifolium (Willdenow) Sprengel. Quim. Nov. 2005, 28, 57–60. [Google Scholar] [CrossRef]
- Achenbach, H.; Blümm, E.; Waibel, R. Vitixanthin and dihydrovitixanthin - new unusual 7′-apocarotenoic acids from. Tetrahedron Lett. 1989, 30, 3059–3060. [Google Scholar] [CrossRef]
- Dixit, B.S.; Srivastava, S.N. Flavonoids and carotenoids of Cochlospermum vitifolium flowers. Fitoterapia 1992, 63, 270. [Google Scholar]
- López, J.A. Flavonoids in Cochlospermum vitifolium Willd (Cochlospermaceae). Ing. Cienc. Quim. 1981, 5, 101–102. [Google Scholar]
- Esposito-avella, M.; Brown, P.; Tejeira, I.; Buitrago, R.; Barrios, L.; Sanchez, C.; Gupta, M.P.; Cedeño, J. Pharmacological screening of Panamanian medicinal plants. Part 1. Int. J. Crude Drug Res. 1985, 23, 17–25. [Google Scholar] [CrossRef]
- Zamora-Martinez, M.C.; de Pascual Pola, C.N. Medicinal plants used in some rural populations of Oaxaca, Puebla and Veracruz, Mexico. J. Ethnopharmacol. 1992, 35, 229–257. [Google Scholar] [CrossRef]
- Monroy-Ortiz, C.; Castillo-España, P. Plantas Medicinales Utilizadas en el Estado de Morelos, 2nd ed.; Universidad Autónoma del Estado de Morelos: Cuernavaca, México, 2007; ISBN 968-878-277-7. [Google Scholar]
- Banos, G.; Perez-Torres, I.; El Hafidi, M. Medicinal agents in the metabolic syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salgado, J.C.; Ortiz-Andrade, R.R.; Aguirre-Crespo, F.; Vergara-Galicia, J.; León-Rivera, I.; Montes, S.; Villalobos-Molina, R.; Estrada-Soto, S. Hypoglycemic, vasorelaxant and hepatoprotective effects of Cochlospermum vitifolium (Willd.) Sprengel: A potential agent for the treatment of metabolic syndrome. J. Ethnopharmacol. 2007, 109, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salgado, J.C.; Castillo-España, P.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Estrada-Soto, S. Cochlospermum vitifolium induces vasorelaxant and antihypertensive effects mainly by activation of NO/cGMP signaling pathway. J. Ethnopharmacol. 2010, 130, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Caballero-George, C.; Vanderheyden, P.M.L.; Solis, P.N.; Pieters, L.; Shahat, A.A.; Gupta, M.P.; Vauquelin, G.; Vlietinck, A.J. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine 2001, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Andrade, R.; Torres-Piedra, M.; Sánchez-Salgado, J.C.; García-Jiménez, S.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Gallardo-Ortíz, I.; Estrada-Soto, S. Acute and sub-chronic effects of Cochlospermum vitifolium in blood glucose levels in normoglycemic and STZ-nicotinamide-induced diabetic rats. Rev. Latinoamer. Quím. 2009, 37, 122–132. [Google Scholar]
- Deharo, E.; Baelmans, R.; Gimenez, A.; Quenevo, C.; Bourdy, G. In vitro immunomodulatory activity of plants used by the Tacana ethnic group in Bolivia. Phytomedicine 2004, 11, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Recillas, A.; Mantecón-Reyes, P.; Castillo-España, P.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Estrada-Soto, S. Tracheal relaxation of five medicinal plants used in Mexico for the treatment of several diseases. Asian Pac. J. Trop. Med. 2014, 7, 179–183. [Google Scholar] [CrossRef]
- HEPAMAP A Roadmap for Hepatology Research in Europe: An Overview for Policy Makers. Available online: www.easl.eu/medias/EASLimg/News/3f9dd90221ef292_file.pdf (accessed on 10 July 2018).
- American Liver Foundation The Liver Lowdown–Liver Disease: The Big Picture. Available online: https://liverfoundation.org/for-patients/resources/liver-lowdown/ (accessed on 10 July 2018).
- Wang, F.-S.; Fan, J.-G.; Zhang, Z.; Gao, B.; Wang, H.-Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-S.; Yang, H.J.; Lee, J.-Y.; Na, Y.-C.; Kwon, S.-Y.; Kim, Y.-C.; Lee, J.-H.; Jang, H.-J. Effects of β-sitosterol derived from Artemisia capillaris on the activated human hepatic stellate cells and dimethylnitrosamine-induced mouse liver fibrosis. BMC Complement. Altern. Med. 2014, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-S.; Chen, J.-H.; Leong, P.-K.; Leung, H.-Y.; Chan, W.-M.; Ko, K.-M. β-Sitosterol protects against carbon tetrachloride hepatotoxicity but not Gentamicin nephrotoxicity in rats via the induction of mitochondrial glutathione redox cycling. Molecules 2014, 19, 17649–17662. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Li, X.-F.; Kang, K.-H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Nafees, S.; Ahmad, S.T.; Arjumand, W.; Rashid, S.; Ali, N.; Sultana, S. Modulatory effects of gentisic acid against genotoxicity and hepatotoxicity induced by cyclophosphamide in Swiss albino mice. J. Pharm. Pharmacol. 2012, 64, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Geng, C.-A.; Ma, Y.-B.; Zhang, X.-M.; Chen, J.-J. Three new secoiridoids, swermacrolactones A–C and anti-hepatitis B virus activity from Swertia macrosperma. Fitoterapia 2013, 89, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Gerin, F.; Erman, H.; Erboga, M.; Sener, U.; Yilmaz, A.; Seyhan, H.; Gurel, A. The effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation 2016, 39, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Pan, Z.; Dong, M.; Yu, C.; Niu, Y. Ferulic acid suppresses activation of hepatic stellate cells through ERK1/2 and Smad signaling pathways in vitro. Biochem. Pharmacol. 2015, 93, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.T.; Jin, X.; Hong, Y.-S.; Lee, J.J. An isoaurone and other constituents from trichosanthes kirilowii seeds inhibit hypoxia-inducible factor-1 and nuclear factor-κB. J. Nat. Prod. 2010, 73, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-R.; Liu, C.-J.; Yeh, C.-C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Kim, N.H.; Kim, J.-Y.; Park, J.-H.; Shin, S.-Y.; Kwon, Y.-S.; Lee, H.J.; Kim, S.-S.; Chun, W. Aromadendrin inhibits lipopolysaccharide-induced nuclear translocation of NF-κB and phosphorylation of JNK in RAW 264.7 macrophage cells. Biomol. Ther. 2013, 21, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, A.; Miyata, S.; Iwase, M.; Shimizu, M.; Inoue, J.; Sato, R. Kaempferol stimulates gene expression of low-density lipoprotein receptor through activation of Sp1 in cultured hepatocytes. Sci. Rep. 2016, 6, 24940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Chen, J.; Zhu, P.; Fujino, M.; Takahara, T.; Toyama, S.; Tomita, A.; Zhao, L.; Yang, Z.; Hei, M.; et al. Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells. Int. Immunopharmacol. 2015, 28, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, C.-A.; Sun, C.-L.; Ma, Y.-B.; Huang, X.-Y.; Cao, T.-W.; He, K.; Wang, H.; Zhang, X.-M.; Chen, J.-J. Polyacetylenes and anti-hepatitis B virus active constituents from Artemisia capillaris. Fitoterapia 2014, 95, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lee, H.Y.; Chang, C.H.; Namba, T.; Masao, H. Evaluation of the liver protective principles from the root of Cudrania cochinchinensis var. gerontogea. Phyther. Res. 1996, 10, 13–17. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, J.-K.; Choi, J.-H.; Jung, J.-Y.; Oh, W.-Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.-H.; et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride–induced hepatic damage in mice. J. Pharmacol. Sci. 2010, 112, 105–112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 3, 4, 11 and 18 are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Guadarrama, A.B.; Rios, M.Y. Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules 2018, 23, 1952. https://doi.org/10.3390/molecules23081952
Aguilar-Guadarrama AB, Rios MY. Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules. 2018; 23(8):1952. https://doi.org/10.3390/molecules23081952
Chicago/Turabian StyleAguilar-Guadarrama, A. Berenice, and María Yolanda Rios. 2018. "Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity" Molecules 23, no. 8: 1952. https://doi.org/10.3390/molecules23081952
APA StyleAguilar-Guadarrama, A. B., & Rios, M. Y. (2018). Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules, 23(8), 1952. https://doi.org/10.3390/molecules23081952