Hepatoprotective Effects of MHY3200 on High-Fat, Diet-Induced, Non-Alcoholic Fatty Liver Disease in Rats
Abstract
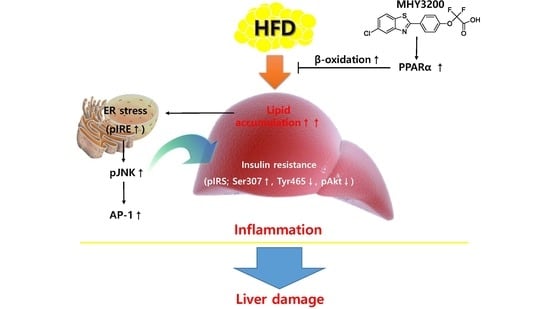
:1. Introduction
2. Results
2.1. Effects of MHY3200 on PPARα Transcriptional Activity
2.2. Biochemical Analyses
2.3. Effects of MHY3200 on Hepatic Lipid Accumulation in the HFD Rat Liver
2.4. Effects of MHY3200 on ER Stress, Insulin Signaling and Inflammation in the HFD Rat Liver
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. General
4.2.1. Synthesis of Ethyl 2,2-difluoro-2-(4-formylphenoxy)acetate (Compound 1)
4.2.2. Synthesis of Ethyl 2-(4-(5-chlorobenzo[d]thiazol-2-yl)phenoxy)-2,2-difluoroacetate (Compound 2)
4.2.3. Synthesis of 2-(4-(5-chlorobenzo[d]thiazol-2-yl)phenoxy)-2,2-difluoroacetic Acid (MHY3200)
4.3. Cell Culture System
4.4. Luciferase Assay
4.5. Animal Experiments
4.6. Serum Biochemical Analyses
4.7. Hepatic TG Measurement
4.8. Tissue Protein Extraction
4.9. Western Blot Analysis
4.10. Isolation and Quantitative Real-Time PCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.L.; Lazo, M.; Selvin, E.; Clark, J.M. Racial differences in nonalcoholic fatty liver disease in the U.S. population. Obesity 2014, 22, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Poonawala, A.; Nair, S.P.; Thuluvath, P.J. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: A case-control study. Hepatology 2000, 32, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Sanchez, N.; Arrese, M.; Zamora-Valdes, D.; Uribe, M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007, 27, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [PubMed]
- Salvado, L.; Palomer, X.; Barroso, E.; Vazquez-Carrera, M. Targeting endoplasmic reticulum stress in insulin resistance. Trends Endocrinol. Metab. 2015, 26, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, C.M.; Titus, E.A.; Cooper, A.A. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 2004, 15, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.T.; Davis, R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)—From inflammation to development. Curr. Opin. Cell Biol. 1998, 10, 205–219. [Google Scholar] [CrossRef]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Jay, M.A.; Ren, J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr. Diabetes Rev. 2007, 3, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Sun, R.Q.; Zeng, X.Y.; Choong, Z.H.; Wang, H.; Watt, M.J.; Ye, J.M. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose–fed mice despite increased endoplasmic reticulum stress. Diabetes 2013, 62, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Park, J.Y.; Lee, H.J.; Kim, D.H.; Park, D.; Jeong, H.O.; Park, C.H.; Chun, P.; Moon, H.R.; Chung, H.Y. Potent anti-diabetic effects of MHY908, a newly synthesized PPAR alpha/gamma dual agonist in db/db mice. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, B.; An, H.J.; Kim, D.H.; Park, K.C.; Noh, S.G.; Chung, K.W.; Lee, E.K.; Kim, K.M.; Kim, D.H.; et al. Novel PPARalpha agonist MHY553 alleviates hepatic steatosis by increasing fatty acid oxidation and decreasing inflammation during aging. Oncotarget 2017, 8, 46273–46285. [Google Scholar] [PubMed]
- Guan, Y.; Zhang, Y.; Breyer, M.D. The role of PPARs in the transcriptional control of cellular processes. Drug News Perspect. 2002, 15, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53 (Suppl. 1), S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Barbuio, R.; Milanski, M.; Bertolo, M.B.; Saad, M.J.; Velloso, L.A. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J. Endocrinol. 2007, 194, 539–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 79, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, D.H.; Kim, M.J.; Lee, E.K.; An, H.J.; Jeong, J.W.; Kim, H.R.; Kim, S.J.; Yu, B.P.; Moon, H.R.; et al. Effects of MHY908, a new synthetic PPARalpha/gamma dual agonist, on inflammatory responses and insulin resistance in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Lonardo, A.; Mussi, C.; Baldelli, E.; Pellegrini, E.; Ballestri, S.; Romagnoli, D.; Loria, P. Nonalcoholic fatty liver disease and aging: Epidemiology to management. World J. Gastroenterol. 2014, 20, 14185–14204. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.; Siersbaek, M.; Mandrup, S. PPARs: Fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012, 23, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W.; Vermeulen, L.; Delerive, P.; De Bosscher, K.; Staels, B.; Haegeman, G. A paradigm for gene regulation: Inflammation, NF-kappaB and PPAR. Adv. Exp. Med. Biol. 2003, 544, 181–196. [Google Scholar] [PubMed]
- Gershoni, J.M.; Palade, G.E. Protein blotting: Principles and applications. Anal. Biochem. 1983, 131, 1–15. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (MHY3200) are available from the authors. |






| HFD | ||||||
|---|---|---|---|---|---|---|
| Item | Chow | Con | 1 mg | 2 mg | ||
| Bodyweight | ||||||
| Initial (g) | 289.3 ± 2.7 | 289.5 ± 3.5 | 290.2 ± 4.2 | 286.3 ± 2.8 | ||
| Final (g) | 448 ± 9.2 a | 538.3 ± 18.1 | 506.2 ± 9.2 | 515 ± 15.5 | ||
| Gain (g) | 158.7 ± 8.8 c | 250.1 ± 21.1 | 216 ± 8 | 228.7 ± 14.5 | ||
| Food Intake (g/days) | 22.2 ± 1.4 | 18.5 ± 1.4 | 20.3 ± 1.7 | 19.6 ± 1.6 | ||
| Water Intake (mL/day) | 51.7 ± 12.9 | 69.1 ± 16.1 | 44.8 ± 12.3 | 79.3 ± 14.9 | ||
| Serum ALT (IU/L) | 1.9 ± 0.5 b | 3.7 ± 0.3 | 1.7 ± 0.2 c | 1.7 ± 0.2 c | ||
| Serum AST (IU/L) | 36.6 ± 3.2 | 39.1 ± 1.1 | 26.8 ± 3.4 | 25.5 ± 2.3 | ||
| Serum Triglyceride (mg/dL) | 61.1± 1.6 a | 81.2 ± 7.7 | 59.8 ± 5.1 a | 53.3 ± 2.1 b | ||
| Serum Cholesterol (mg/dL) | 96.9 ± 4.9 c | 207.8 ± 11.2 | 162 ± 16.9 b | 317.9 ± 32.3 a | ||
| Serum FFA (uEq/L) | 427 ± 32.1 b | 644 ± 20 | 627 ± 55.1 | 548.6 ± 28.9 | ||
| Serum Glucose (mg/dL) | 91.8 ± 3.1 a | 103.3 ± 2.29 | 95.3 ± 2.17 | 91 ± 2.9 a | ||
| Serum Insulin (ng/mL) | 1.3 ± 0.08 a | 2.1 ± 0.24 | 1.3 ± 0.13 a | 0.99 ± 0.05 b | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Park, C.H.; Kim, D.H.; Park, M.H.; Park, K.C.; Hyun, M.K.; Lee, A.K.; Moon, H.R.; Chung, H.Y. Hepatoprotective Effects of MHY3200 on High-Fat, Diet-Induced, Non-Alcoholic Fatty Liver Disease in Rats. Molecules 2018, 23, 2057. https://doi.org/10.3390/molecules23082057
Kim MJ, Park CH, Kim DH, Park MH, Park KC, Hyun MK, Lee AK, Moon HR, Chung HY. Hepatoprotective Effects of MHY3200 on High-Fat, Diet-Induced, Non-Alcoholic Fatty Liver Disease in Rats. Molecules. 2018; 23(8):2057. https://doi.org/10.3390/molecules23082057
Chicago/Turabian StyleKim, Min Jo, Chan Hum Park, Dae Hyun Kim, Min Hi Park, Kyung Chul Park, Min Kyung Hyun, A Kyoung Lee, Hyung Ryong Moon, and Hae Young Chung. 2018. "Hepatoprotective Effects of MHY3200 on High-Fat, Diet-Induced, Non-Alcoholic Fatty Liver Disease in Rats" Molecules 23, no. 8: 2057. https://doi.org/10.3390/molecules23082057
APA StyleKim, M. J., Park, C. H., Kim, D. H., Park, M. H., Park, K. C., Hyun, M. K., Lee, A. K., Moon, H. R., & Chung, H. Y. (2018). Hepatoprotective Effects of MHY3200 on High-Fat, Diet-Induced, Non-Alcoholic Fatty Liver Disease in Rats. Molecules, 23(8), 2057. https://doi.org/10.3390/molecules23082057