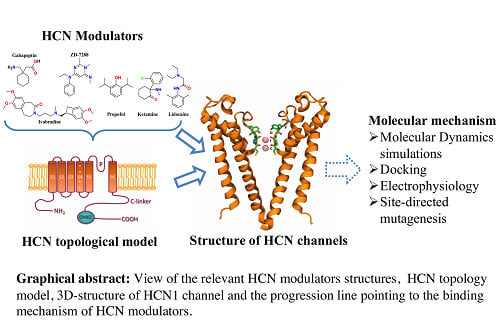
HCN Channels: New Therapeutic Targets for Pain Treatment
Abstract
:1. Introduction
HCN Channels Structure and Function
2. HCN Channel Regulation
3. HCN Channels in the Central Nervous System
4. The Role of HCN Channels in Pain Perception
5. HCN Channels as a Pharmacological Target for Analgesia
6. Challenges and Future Directions in Structure-Based Drug Design Targeting HCN Channels
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gauss, R.; Seifert, R.; Kaupp, U.B. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 1998, 393, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Zong, X.; Jeglitsch, M.; Hofmann, F.; Biel, M. A family of hyperpolarization-activated mammalian cation channels. Nature 1998, 393, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Santoro, B.; Liu, D.T.; Yao, H.; Bartsch, D.; Kandel, E.R.; Siegelbaum, S.A.; Tibbs, G.R. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 1998, 93, 717–729. [Google Scholar] [CrossRef]
- Lee, C.-H.; MacKinnon, R. Structures of the human HCN1 hyperpolarization-activated channel. Cell 2017, 168, 111–120. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D.; Tortora, P. Direct activation of cardiac pacemaker channels by intracellular cyclic amp. Nature 1991, 351, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Nolan, M.F.; Siegelbaum, S.A. Activity-dependent regulation of HCN pacemaker channels by cyclic amp. Neuron 2002, 36, 451–461. [Google Scholar] [CrossRef]
- Biel, M.; Wahl-Schott, C.; Michalakis, S.; Zong, X. Hyperpolarization-activated cation channels: From genes to function. Physiol. Rev. 2009, 89, 847–885. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, M.R.; Schwabe, T.; Ehrlich, G.; Kusch, J.; Benndorf, K. All four subunits of HCN2 channels contribute to the activation gating in an additive but intricate manner. J. Gen. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Stieber, J.; Thomer, A.; Much, B.; Schneider, A.; Biel, M.; Hofmann, F. Molecular basis for the different activation kinetics of the pacemaker channels HCN2 and HCN4. J. Biol. Chem. 2003, 278, 33672–33680. [Google Scholar] [CrossRef] [PubMed]
- Wainger, B.J.; DeGennaro, M.; Santoro, B.; Siegelbaum, S.A.; Tibbs, G.R. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 2001, 411, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Stieber, J.; Stöckl, G.; Herrmann, S.; Hassfurth, B.; Hofmann, F. Functional expression of the human HCN3 channel. J. Biol. Chem. 2005, 280, 34635–34643. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-W.; Tibbs, G.R.; Picollo, A.; Abbas, S.Y.; Sanford, R.L.; Accardi, A.; Hofmann, F.; Ludwig, A.; Goldstein, P.A. Pip2-mediated HCN3 channel gating is crucial for rhythmic burst firing in thalamic intergeniculate leaflet neurons. J. Neurosci. 2011, 31, 10412–10423. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.W.; Garthwaite, J. Hyperpolarization-activated ion channels as targets for nitric oxide signalling in deep cerebellar nuclei. Eur. J. Neurosci. 2010, 31, 1935–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusch, J.; Biskup, C.; Thon, S.; Schulz, E.; Nache, V.; Zimmer, T.; Schwede, F.; Benndorf, K. Interdependence of receptor activation and ligand binding in HCN2 pacemaker channels. Neuron 2010, 67, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kusch, J.; Thon, S.; Schulz, E.; Biskup, C.; Nache, V.; Zimmer, T.; Seifert, R.; Schwede, F.; Benndorf, K. How subunits cooperate in cAMP-induced activation of homotetrameric HCN2 channels. Nat. Chem. Biol. 2011, 8, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Frère, S.G.; Kuisle, M.; Lüthi, A. Regulation of recombinant and native hyperpolarization-activated cation channels. Mol. Neurobiol. 2004, 30, 279–305. [Google Scholar] [CrossRef]
- Much, B.; Wahl-Schott, C.; Zong, X.; Schneider, A.; Baumann, L.; Moosmang, S.; Ludwig, A.; Biel, M. Role of subunit heteromerization and n-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J. Biol. Chem. 2003, 278, 43781–43786. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 2000, 80, 555–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mitcheson, J.S.; Lin, M.; Sanguinetti, M.C. Functional roles of charged residues in the putative voltage sensor of the HCN2 pacemaker channel. J. Biol. Chem. 2000, 275, 36465–36471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ruta, V.; Chen, J.; Lee, A.; MacKinnon, R. The principle of gating charge movement in a voltage-dependent k+ channel. Nature 2003, 423, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vaca, L.; Stieber, J.; Zong, X.; Ludwig, A.; Hofmann, F.; Biel, M. Mutations in the s4 domain of a pacemaker channel alter its voltage dependence. FEBS Lett. 2000, 479, 35–40. [Google Scholar] [CrossRef]
- Zhou, Y.; Morais-Cabral, J.H.; Kaufman, A.; MacKinnon, R. Chemistry of ion coordination and hydration revealed by a k+ channel-fab complex at 2.0 a resolution. Nature 2001, 414, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Duan, K.-L.; Shang, C.-F.; Yu, H.-G.; Zhou, Z. Calcium influx through hyperpolarization-activated cation channels (Ih channels) contributes to activity-evoked neuronal secretion. Proc. Natl. Acad. Sci. USA 2004, 101, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, X.-W.; Zhou, P.; Yao, L.; Liu, T.; Zhang, B.; Li, Y.; Zheng, H.; Zheng, L.-H.; Zhang, C.X.; et al. Calcium influx through if channels in rat ventricular myocytes. Am. J. Physiol. Cell Physiol. 2007, 292, C1147–C1155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Neher, E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. Eur. J. Physiol. 1993, 425, 511–517. [Google Scholar] [CrossRef]
- Schneggenburger, R.; Zhou, Z.; Konnerth, A.; Neher, E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron 1993, 11, 133–143. [Google Scholar] [CrossRef]
- Burnashev, N.; Zhou, Z.; Neher, E.; Sakmann, B. Fractional calcium currents through recombinant glur channels of the nmda, ampa and kainate receptor subtypes. J. Physiol. 1995, 485, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, C.; Hagen, V.; Kaupp, U.B.; Frings, S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J. 1999, 18, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bers, D.M. Ca2+ influx via the l-type ca2+ channel during tail current and above current reversal potential in ferret ventricular myocytes. J. Physiol. 2000, 523, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Craven, K.B.; Zagotta, W.N. Cng and HCN channels: Two peas, one pod. Annu. Rev. Physiol. 2006, 68, 375–401. [Google Scholar] [CrossRef] [PubMed]
- Wahl-Schott, C.; Biel, M. HCN channels: Structure, cellular regulation and physiological function. Cell. Mol. Life Sci. 2009, 66, 470–494. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, W.N.; Olivier, N.B.; Black, K.D.; Young, E.C.; Olson, R.; Gouaux, E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 2003, 425, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Craven, K.B.; Olivier, N.B.; Zagotta, W.N. C-terminal movement during gating in cyclic nucleotide-modulated channels. J. Biol. Chem. 2008, 283, 14728–14738. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, M.; Nardini, M.; Gazzarrini, S.; Möller, S.; Bertinetti, D.; Herberg, F.W.; Bolognesi, M.; Martin, H.; Fasolini, M.; Bertrand, J.A.; et al. Tetramerization dynamics of C-terminal domain underlies isoform-specific cAMP gating in hyperpolarization-activated cyclic nucleotide-gated channels. J. Biol. Chem. 2011, 286, 44811–44820. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, M.; Bucchi, A.; Arrigoni, C.; Zucca, S.; Nardini, M.; Schroeder, I.; Simmons, K.; Aquila, M.; DiFrancesco, D.; Bolognesi, M.; et al. Cyclic dinucleotides bind the C-linker of HCN4 to control channel cAMP responsiveness. Nat. Chem. Biol. 2014, 10, 457–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, S.; Alfieri, A.; Bertinetti, D.; Aquila, M.; Schwede, F.; Lolicato, M.; Rehmann, H.; Moroni, A.; Herberg, F.W. Cyclic nucleotide mapping of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. ACS Chem. Biol. 2014, 9, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Taraska, J.W.; Puljung, M.C.; Olivier, N.B.; Flynn, G.E.; Zagotta, W.N. Mapping the structure and conformational movements of proteins with transition metal ion fret. Nat. Methods 2009, 6, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; You, Z.; Shen, S.; Chen, L.; Zhu, S.; Mao, J. Inhibition of HCN channel activity in the thalamus attenuates chronic pain in rats. Neurosci. Lett. 2016, 631, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Shigemoto, R. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J. Comp. Neurol. 2004, 471, 241–276. [Google Scholar] [CrossRef] [PubMed]
- Moosmang, S.; Biel, M.; Hofmann, F.; Ludwig, A. Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biol. Chem. 1999, 380, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Moosmang, S.; Stieber, J.; Zong, X.; Biel, M.; Hofmann, F.; Ludwig, A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur. J. Biochem. 2001, 268, 1646–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, B.; Chen, S.; Lüthi, A.; Pavlidis, P.; Shumyatsky, G.P.; Tibbs, G.R.; Siegelbaum, S.A. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse cns. J. Neurosci. 2000, 20, 5264–5275. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Zong, X.; Stieber, J.; Hullin, R.; Hofmann, F.; Biel, M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999, 18, 2323–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Chen, F.; Li, B.; Hu, Z. Neurophysiology of HCN channels: From cellular functions to multiple regulations. Prog. Neurobiol. 2014, 112, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shu, S.; Bayliss, D.A. Suppression of Ih contributes to propofol-induced inhibition of mouse cortical pyramidal neurons. J. Neurophysiol. 2005, 94, 3872–3883. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.F.; Malleret, G.; Lee, K.H.; Gibbs, E.; Dudman, J.T.; Santoro, B.; Yin, D.; Thompson, R.F.; Siegelbaum, S.A.; Kandel, E.R.; et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar purkinje cells. Cell 2003, 115, 551–564. [Google Scholar] [CrossRef]
- Ying, S.-W.; Abbas, S.Y.; Harrison, N.L.; Goldstein, P.A. Propofol block of Ih contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur. J. Neurosci. 2006, 23, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Chang, P.Y.; Johnston, D. Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors. Neuron 2012, 75, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Harnett, M.T.; Morikawa, H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J. Neurophysiol. 2006, 95, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Simkin, D.; Suyeoka, G.M.; Chetkovich, D.M. Evaluation of HCN2 abnormalities as a cause of juvenile audiogenic seizures in black swiss mice. Brain Res. 2006, 1083, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Descalzi, G.; Chen, T.; Ko, H.-G.; Lu, J.; Li, S.; Son, J.; Kim, T.; Kwak, C.; Huganir, R.L.; et al. Coexistence of two forms of ltp in acc provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015, 85, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, S.-J.; Cui, J.; He, W.-J.; Ruan, H.-Z. The role of HCN channels within the periaqueductal gray in neuropathic pain. Brain Res. 2013, 1500, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Schnorr, S.; Ludwig, A. HCN channels—Modulators of cardiac and neuronal excitability. Int. J. Mol. Sci. 2015, 16, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Munsch, T.; Pape, H.C. Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. J. Physiol. 1999, 519, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pian, P.; Bucchi, A.; Robinson, R.B.; Siegelbaum, S.A. Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous Pip2. J. Gen. Physiol. 2006, 128, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Zolles, G.; Klöcker, N.; Wenzel, D.; Weisser-Thomas, J.; Fleischmann, B.K.; Roeper, J.; Fakler, B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 2006, 52, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Stieber, J.; Ludwig, A.; Hofmann, F.; Biel, M. A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. J. Biol. Chem. 2001, 276, 6313–6319. [Google Scholar] [CrossRef] [PubMed]
- Arinsburg, S.S.; Cohen, I.S.; Yu, H.-G. Constitutively active src tyrosine kinase changes gating of HCN4 channels through direct binding to the channel proteins. J. Cardiovasc. Pharmacol. 2006, 47, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Zhang, Q.; Teng, B.; Mustafa, S.J.; Huang, J.-Y.; Yu, H.-G. Src tyrosine kinase alters gating of hyperpolarization-activated HCN4 pacemaker channel through Tyr531. Am. J. Physiol. Cell Physiol. 2008, 294, C355–C362. [Google Scholar] [CrossRef] [PubMed]
- Fogle, K.J.; Lyashchenko, A.K.; Turbendian, H.K.; Tibbs, G.R. HCN pacemaker channel activation is controlled by acidic lipids downstream of diacylglycerol kinase and phospholipase A2. J. Neurosci. 2007, 27, 2802–2814. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bunney, E.B.; Appel, S.B.; Brodie, M.S. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: Involvement of 5- CA1 receptors and protein kinase C. J. Neurophysiol. 2003, 90, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Reetz, O.; Strauss, U. Protein kinase c activation inhibits rat and human hyperpolarization activated cyclic nucleotide gated channel (HCN)1—Mediated current in mammalian cells. Cell. Physiol. Biochem. 2013, 31, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Fürst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C.; Carruth, M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J. Neurophysiol. 1999, 82, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 1999, 2, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 2000, 1, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C. Dendritic mechanisms of phase precession in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 2001, 86, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wymore, R.; Yu, H.; Wu, J.; Wymore, R.T.; Pan, Z.; Robinson, R.B.; Dixon, J.E.; McKinnon, D.; Cohen, I.S. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mrna expression in cardiac tissues. Circ. Res. 1999, 85, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Stieber, J.; Hofmann, F.; Ludwig, A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc. Med. 2004, 14, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lujan, R.; Albasanz, J.L.; Shigemoto, R.; Juiz, J.M. Preferential localization of the hyperpolarization-activated cyclic nucleotide-gated cation channel subunit HCN1 in basket cell terminals of the rat cerebellum. Eur. J. Neurosci. 2005, 21, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, R.; Gonzalez, D.; Valenzuela, C.; Brown, N.; Zuniga, L. Expression and cellular localization of HCN channels in rat cerebellar granule neurons. Biochem. Biophys. Res. Commun. 2016, 478, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. HCN channels: Function and clinical implications. Neurology 2013, 80, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Lantéri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Torrance, N.; Smith, B.H. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Tibbs, G.R.; Posson, D.J.; Goldstein, P.A. Voltage-gated ion channels in the pns: Novel therapies for neuropathic pain? Trends Pharmacol. Sci. 2016, 37, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Guo, H.-Q.; Lee, D.H.; Luo, L.; Liu, C.; Kuei, C.; Velumian, A.A.; Butler, M.P.; Brown, S.M.; Dubin, A.E. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J. Neurosci. 2003, 23, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Baruscotti, M.; Bottelli, G.; Milanesi, R.; DiFrancesco, J.C.; DiFrancesco, D. HCN-related channelopathies. Pflugers Arch. Eur. J. Physiol. 2010, 460, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Young, G.-T.; Berrocoso, E.M.; Chen, L.; McNaughton, P.A. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011, 333, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Smith, T.; Sathish, J.; Djouhri, L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not a δ-nociceptors. Pain 2012, 153, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Young, G.T.; McNaughton, P.A. HCN2 ion channels: An emerging role as the pacemakers of pain. Trends Pharmacol. Sci. 2012, 33, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Young, G.T.; Emery, E.C.; Mooney, E.R.; Tsantoulas, C.; McNaughton, P.A. Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain 2014, 155, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Deng, L.; Sun, Q.; Yao, L.; Han, J.-S.; Wan, Y. Hyperpolarization-activated, cyclic nucleotide-gated cation channels: Roles in the differential electrophysiological properties of rat primary afferent neurons. J. Neurosci. Res. 2004, 76, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chang, L.; Brown, S.M.; Ao, H.; Lee, D.H.; Higuera, E.S.; Dubin, A.E.; Chaplan, S.R. Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience 2007, 144, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Momin, A.; Cadiou, H.; Mason, A.; McNaughton, P.A. Role of the hyperpolarization-activated current Ih in somatosensory neurons. J. Physiol. 2008, 586, 5911–5929. [Google Scholar] [CrossRef] [PubMed]
- Tsantoulas, C.; Laínez, S.; Wong, S.; Mehta, I.; Vilar, B.; McNaughton, P.A. Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.K.; Nones, C.F.; Reis, R.C.; Chichorro, J.G.; Cunha, J.M. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 2015, 6, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Aromolaran, K.A.; Goldstein, P.A. Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy: Cause and effect? Mol. Pain 2017, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Descoeur, J.; Pereira, V.; Pizzoccaro, A.; Francois, A.; Ling, B.; Maffre, V.; Couette, B.; Busserolles, J.; Courteix, C.; Noel, J.; et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011, 3, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, V.; Busserolles, J.; Christin, M.; Devilliers, M.; Poupon, L.; Legha, W.; Alloui, A.; Aissouni, Y.; Bourinet, E.; Lesage, F.; et al. Role of the trek2 potassium channel in cold and warm thermosensation and in pain perception. Pain 2014, 155, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Resta, F.; Micheli, L.; Laurino, A.; Spinelli, V.; Mello, T.; Sartiani, L.; Di Cesare Mannelli, L.; Cerbai, E.; Ghelardini, C.; Romanelli, M.N.; et al. Selective HCN1 block as a strategy to control oxaliplatin-induced neuropathy. Neuropharmacology 2018, 131, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieber, J.; Wieland, K.; Stöckl, G.; Ludwig, A.; Hofmann, F. Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol. Pharmacol. 2006, 69, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dougherty, P.M. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology 2014, 120, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Hagains, C.E.; Senapati, A.K.; Huntington, P.J.; He, J.-W.; Peng, Y.B. Inhibition of spinal cord dorsal horn neuronal activity by electrical stimulation of the cerebellar cortex. J. Neurophysiol. 2011, 106, 2515–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuner, R. Central mechanisms of pathological pain. Nat. Med. 2010, 16, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Tsantoulas, C.; McMahon, S.B. Opening paths to novel analgesics: The role of potassium channels in chronic pain. Trends Neurosci. 2014, 37, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Ono, H.; Tanabe, M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain. Pain 2010, 151, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xing, G.-G.; Tu, H.-Y.; Han, J.-S.; Wan, Y. Inhibition of hyperpolarization-activated current by ZD7288 suppresses ectopic discharges of injured dorsal root ganglion neurons in a rat model of neuropathic pain. Brain Res. 2005, 1032, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Staikopoulos, V.; Furness, J.B.; Jennings, E.A. Inflammation-induced increase in hyperpolarization-activated, cyclic nucleotide-gated channel protein in trigeminal ganglion neurons and the effect of buprenorphine. Neuroscience 2009, 162, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; You, Z.; Shen, S.; Yang, J.; Lim, G.; Doheny, J.T.; Zhu, S.; Zhang, Y.; Chen, L.; Mao, J. Increased HCN channel activity in the gasserian ganglion contributes to trigeminal neuropathic pain. J. Pain 2018, 19, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Al Otaibi, M.; Sathish, J.; Djouhri, L. Increased expression of HCN2 channel protein in L4 dorsal root ganglion neurons following axotomy of L5- and inflammation of L4-spinal nerves in rats. Neuroscience 2015, 295, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Tibbs, G.R.; Rowley, T.J.; Sanford, R.L.; Herold, K.F.; Proekt, A.; Hemmings, H.C.; Andersen, O.S.; Goldstein, P.A.; Flood, P.D. HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain. J. Pharmacol. Exp. Ther. 2013, 345, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; You, Z.; Wang, S.; Yang, J.; Yang, L.; Sun, Y.; Mi, W.; Yang, L.; McCabe, M.F.; Shen, S.; et al. Neuropeptide s modulates the amygdaloidal HCN activities (Ih) in rats: Implication in chronic pain. Neuropharmacology 2016, 105, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, S.J.; Cui, J.; He, W.J.; Ruan, H.Z. Inhibition of HCN channels within the periaqueductal gray attenuates neuropathic pain in rats. Behav. Neurosci. 2013, 127, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro Matos, S.; Zhang, Z.; Seguela, P. Peripheral neuropathy induces HCN channel dysfunction in pyramidal neurons of the medial prefrontal cortex. J. Neurosci. 2015, 35, 13244–13256. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Kumar, N.; Bukhanova, N.; Chen, Y.; Stemkowsi, P.L.; Smith, P.A. The heart-rate-reducing agent, ivabradine, reduces mechanical allodynia in a rodent model of neuropathic pain. Eur. J. Pain 2014, 18, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Surges, R.; Freiman, T.M.; Feuerstein, T.J. Gabapentin increases the hyperpolarization-activated cation current Ih in rat CA1 pyramidal cells. Epilepsia 2003, 44, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tae, H.-S.; Smith, K.M.; Phillips, A.M.; Boyle, K.A.; Li, M.; Forster, I.C.; Hatch, R.J.; Richardson, R.; Hughes, D.I.; Graham, B.A.; et al. Gabapentin modulates HCN4 channel voltage-dependence. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Postea, O.; Biel, M. Exploring HCN channels as novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, A.; Baruscotti, M.; Nardini, M.; Barbuti, A.; Micheloni, S.; Bolognesi, M.; DiFrancesco, D. Identification of the molecular site of ivabradine binding to HCN4 channels. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, M.; Del Lungo, M.; Guandalini, L.; Martini, E.; Dei, S.; Manetti, D.; Scapecchi, S.; Teodori, E.; Sartiani, L.; Mugelli, A.; et al. Design, synthesis, and preliminary biological evaluation of new isoform-selective f-current blockers. J. Med. Chem. 2010, 53, 6773–6777. [Google Scholar] [CrossRef] [PubMed]
- De Lungo, M.; Melchiorre, M.; Guandalini, L.; Sartiani, L.; Mugelli, A.; Koncz, I.; Szel, T.; Varro, A.; Romanelli, M.N.; Cerbai, E. Novel blockers of hyperpolarization-activated current with isoform selectivity in recombinant cells and native tissue. Br. J. Pharmacol. 2012, 166, 602–616. [Google Scholar] [CrossRef] [PubMed]
- McClure, K.J.; Maher, M.; Wu, N.; Chaplan, S.R.; Eckert, W.A.; Lee, D.H.; Wickenden, A.D.; Hermann, M.; Allison, B.; Hawryluk, N.; et al. Discovery of a novel series of selective HCN1 blockers. Bioorg. Med. Chem. Lett. 2011, 21, 5197–5201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Kinard, K.; Rajamani, R.; Sanguinetti, M.C. Molecular mapping of the binding site for a blocker of hyperpolarization-activated, cyclic nucleotide-modulated pacemaker channels. J. Pharmacol. Exp. Ther. 2007, 322, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Carloni, P. Molecular modeling of ion channels: Structural predictions. Curr. Opin. Chem. Biol. 2003, 7, 150–156. [Google Scholar] [CrossRef]
- Giorgetti, A.; Carloni, P.; Mistrik, P.; Torre, V. A homology model of the pore region of HCN channels. Biophys. J. 2005, 89, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Glajch, K.E.; Gertler, T.S.; Guzman, J.N.; Mercer, J.N.; Lewis, A.S.; Goldberg, A.B.; Tkatch, T.; Shigemoto, R.; Fleming, S.M.; et al. HCN channelopathy in external globus pallidus neurons in models of parkinson’s disease. Nat. Neurosci. 2011, 14. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.S.; Chetkovich, D.M. HCN channels in behavior and neurological disease: Too hyper or not active enough? Mol. Cell. Neurosci. 2011, 46, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noam, Y.; Bernard, C.; Baram, T.Z. Towards an integrated view of HCN channel role in epilepsy. Curr. Opin. Neurobiol. 2011, 21, 873–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, E.; Waterhouse, L.; Duflou, J.; Bagnall, R.D.; Semsarian, C. Genetic analysis of hyperpolarization-activated cyclic nucleotide-gated cation channels in sudden unexpected death in epilepsy cases. Brain Pathol. 2011, 21, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Budde, T.; Stieber, J.; Moosmang, S.; Wahl, C.; Holthoff, K.; Langebartels, A.; Wotjak, C.; Munsch, T.; Zong, X.; et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003, 22, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruscotti, M.; Bucchi, A.; Viscomi, C.; Mandelli, G.; Consalez, G.; Gnecchi-Rusconi, T.; Montano, N.; Casali, K.R.; Micheloni, S.; Barbuti, A.; et al. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene HCN4. Proc. Natl. Acad. Sci. USA 2011, 108, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.; Arévalo, B.; Martínez, G.; Rinné, S.; Sepúlveda, F.V.; Decher, N.; González, W. Side fenestrations provide an “anchor” for a stable binding of A1899 to the pore of Task-1 potassium channels. Mol. Pharm. 2017, 14, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D. Computational methods applied to rational drug design. Open Med. Chem. J. 2016, 10, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.A.; Gutiérrez, M.; Ramírez, D.; Alzate-Morales, J.; Bernal, C.C.; Güiza, F.M.; Romero Bohórquez, A.R. Novel n-allyl/propargyl tetrahydroquinolines: Synthesis via three-component cationic imino diels-alder reaction, binding prediction, and evaluation as cholinesterase inhibitors. Chem. Biol. Drug Des. 2016, 88, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Antkowiak, B. Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci. 2004, 5, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008, 9, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Tokimasa, T.; Sugiyama, K.; Akasu, T.; Muteki, T. Volatile anaesthetics inhibit a cyclic AMP-dependent sodium-potassium current in cultured sensory neurones of bullfrog. Br. J. Pharmacol. 1990, 101, 190–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirois, J.E.; Pancrazio, J.J.; Lynch, C.; Bayliss, D.A. Multiple ionic mechanisms mediate inhibition of rat motoneurones by inhalation anaesthetics. J. Physiol. 1998, 512, 851–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirois, J.E.; Lynch, C.; Bayliss, D.A. Convergent and reciprocal modulation of a leak K+ current and Ih by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones. J. Physiol. 2002, 541, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sirois, J.E.; Lei, Q.; Talley, E.M.; Lynch, C.; Bayliss, D.A. HCN subunit-specific and cAMP-modulated effects of anesthetics on neuronal pacemaker currents. J. Neurosci. 2005, 25, 5803–5814. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shu, S.; Kennedy, D.P.; Willcox, S.C.; Bayliss, D.A. Subunit-specific effects of isoflurane on neuronal Ih in HCN1 knockout mice. J. Neurophysiol. 2009, 101, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mattusch, C.; Kratzer, S.; Buerge, M.; Kreuzer, M.; Engel, T.; Kopp, C.; Biel, M.; Hammelmann, V.; Ying, S.-W.; Goldstein, P.A.; et al. Impact of hyperpolarization-activated, cyclic nucleotide-gated cation channel type 2 for the xenon-mediated anesthetic effect: Evidence from in vitro and in vivo experiments. Anesthesiology 2015, 122, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Mathers, D.A.; Puil, E. Pentobarbital modulates intrinsic and gaba-receptor conductances in thalamocortical inhibition. Neuroscience 2003, 121, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shu, S.; Bayliss, D.A. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J. Neurosci. 2009, 29, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Douglas, J.E.; Kumar, N.N.; Shu, S.; Bayliss, D.A.; Chen, X. Forebrain HCN1 channels contribute to hypnotic actions of ketamine. Anesthesiology 2013, 118, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Cacheaux, L.P.; Topf, N.; Tibbs, G.R.; Schaefer, U.R.; Levi, R.; Harrison, N.L.; Abbott, G.W.; Goldstein, P.A. Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J. Pharmacol. Exp. Ther. 2005, 315, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, D.V.; Shan, Q.; Lee, Y.; Mayer, S.C.; Bowlby, M.R.; Strassle, B.W.; Kaftan, E.J.; Rogers, K.E.; Dunlop, J. Direct inhibition of Ih by analgesic loperamide in rat drg neurons. J. Neurophysiol. 2007, 97, 3713–3721. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Vasilyev, D.V.; Shan, Q.J.; Dunlop, J.; Mayer, S.; Bowlby, M.R. Novel pharmacological activity of loperamide and cp-339,818 on human HCN channels characterized with an automated electrophysiology assay. Eur. J. Pharmacol. 2008, 581, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Knaus, A.; Zong, X.; Beetz, N.; Jahns, R.; Lohse, M.J.; Biel, M.; Hein, L. Direct inhibition of cardiac hyperpolarization-activated cyclic nucleotide-gated pacemaker channels by clonidine. Circulation 2007, 115, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Andrews, G.D.; Glen, W.B.; Lavin, A. A2-noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J. Physiol. 2007, 584, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Meng, Q.-T.; Pan, X.; Xia, Z.-Y.; Chen, X.-D. Dexmedetomidine produced analgesic effect via inhibition of HCN currents. Eur. J. Pharmacol. 2014, 740, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, P.A. HCN1 channels as targets for volatile anesthetics: Coming to the fore. Anesth. Analg. 2015, 121, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liang, P.; Liu, J.; Ke, B.; Wang, X.; Li, F.; Li, T.; Bayliss, D.A.; Chen, X. HCN1 channels contribute to the effects of amnesia and hypnosis but not immobility of volatile anesthetics. Anesth. Analg. 2015, 121, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, T.; Yuan, Z.; Wei, Z.; Yamaki, V.N.; Huang, M.; Huganir, R.L.; Cai, X. Essential roles of aMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci. Signal. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Xia, Z.; Liu, J.; Bayliss, D.A.; Chen, X. Local anesthetic inhibits hyperpolarization-activated cationic currents. Mol. Pharmacol. 2011, 79, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, J.; Yu, K.; Zhou, Y.; Jiang, H.; Chen, K.; Liu, H. Current strategies for the discovery of K+ channel modulators. Curr. Top. Med. Chem. 2009, 9, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Coburn, C.A.; Luo, Y.; Cui, M.; Wang, J.; Soll, R.; Dong, J.; Hu, B.; Lyon, M.A.; Santarelli, V.P.; Kraus, R.L.; et al. Discovery of a pharmacologically active antagonist of the two-pore-domain potassium channel K2p9.1 (Task[-3). Chem. Med. Chem. 2012, 7, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C. The process of structure-based drug design. Chem. Biol. 2003, 10, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, C.; Grubmüller, H.; de Groot, B.L.; Zachariae, U. Computational electrophysiology: The molecular dynamics of ion channel permeation and selectivity in atomistic detail. Biophys. J. 2011, 101, 809–817. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, D.; Zúñiga, R.; Concha, G.; Zúñiga, L. HCN Channels: New Therapeutic Targets for Pain Treatment. Molecules 2018, 23, 2094. https://doi.org/10.3390/molecules23092094
Ramírez D, Zúñiga R, Concha G, Zúñiga L. HCN Channels: New Therapeutic Targets for Pain Treatment. Molecules. 2018; 23(9):2094. https://doi.org/10.3390/molecules23092094
Chicago/Turabian StyleRamírez, David, Rafael Zúñiga, Guierdy Concha, and Leandro Zúñiga. 2018. "HCN Channels: New Therapeutic Targets for Pain Treatment" Molecules 23, no. 9: 2094. https://doi.org/10.3390/molecules23092094
APA StyleRamírez, D., Zúñiga, R., Concha, G., & Zúñiga, L. (2018). HCN Channels: New Therapeutic Targets for Pain Treatment. Molecules, 23(9), 2094. https://doi.org/10.3390/molecules23092094