Corrosion Resistance of Aluminum against Acid Activation: Impact of Benzothiazole-Substituted Gallium Phthalocyanine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Open Circuit Potential (OCP) Time Evolution
2.2. Potentiodynamic Polarization Measurements
2.2.1. Corrosion Current Density (icorr)
2.2.2. Corrosion Potential (Ecorr)
2.2.3. Tafel Slopes (βa and βc)
2.2.4. Corrosion Inhibition Efficiency (IE%)
2.3. Adsorption Isotherms
2.4. Electrochemical Impedance Spectroscopy (EIS)
2.5. Surface Analysis
2.5.1. FTIR Spectra
2.5.2. SEM and EDX
2.5.3. X-ray Diffraction Studies
2.6. Quantum Chemical Studies
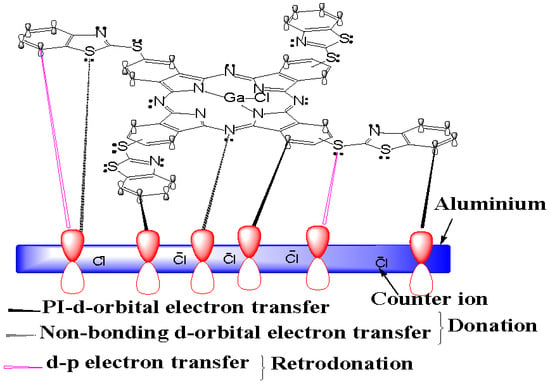
2.7. Inhibition Mechanism
3. Experimental Section
3.1. Materials
3.2. Equipment
3.3. Electrochemical Measurements
3.4. Quantum Chemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oguzie, E.E. Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros. Sci. 2007, 49, 1527–1539. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.M. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim. Acta 2006, 51, 1313–1321. [Google Scholar] [CrossRef]
- Osório, W.R.; Cheung, N.; Peixoto, L.C.; Garcia, A. Corrosion Resistance and Mechanical Properties of an Al 9wt% Si Alloy Treated by Laser Surface Remelting. Int. J. Electrochem. Sci. 2009, 4, 820–831. [Google Scholar]
- El-Shafei, A.A.; Moussa, M.N.H.; El-Far, A.A. Inhibitory effect of amino acids on Al pitting corrosion in 0.1 M NaCl. J. Appl. Electrochem. 1997, 27, 1075–1078. [Google Scholar] [CrossRef]
- Xhanari, K.; Finsgar, M. Organic corrosion inhibitors for aluminium and its alloys in acid solutions: A review. RSC Adv. 2016, 6, 62833–62857. [Google Scholar] [CrossRef]
- El-Meligi, A.A. Corrosion Preventive Strategies as a Crucial Need for Decreasing Environmental Pollution and Saving Economics. Recent Pat. Corres. Sci. 2010, 2, 22–33. [Google Scholar] [CrossRef]
- Nnaji, N.J.N.; Ujam, O.T.; Ibisi, N.E.; Ani, J.U.; Onuegbu, T.O.; Olasunkanmi, L.O.; Ebenso, E.E. Morpholine and piperazine based carboxamide derivatives as corrosion inhibitors of mild steel in HCl medium. J. Mol. Liq. 2017, 230, 652–661. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. Adsorption Characteristics and Corrosion Inhibitive Properties of Clotrimazole for Aluminium Corrosion in Hydrochloric Acid. Int. J. Electrochem. Sci. 2009, 4, 863–877. [Google Scholar]
- Abd El-Maksoud, S.A. The Effect of Organic Compounds on the Electrochemical Behaviour of Steel in Acidic Media. A review. Int. J. Electrochem. Sci. 2008, 3, 528–555. [Google Scholar]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Rehim, S.S.; Refaey, S.A.M.; Taha, F.; Saleh, M.B.; Ahmed, R.A. Corrosion Inhibition of Mild Steel in Acidic Medium using 2-amino Thiophenol and 2-Cyanomethyl Benzothiazole. J. Appl. Electrochem. 2001, 31, 429–435. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, S.; Kumari, N.; Bahadur, I.; Ebenso, E.E. Experimental and Theoretical Studies on Corrosion Inhibition Effect of Synthesized Benzothiazole Derivatives on Mild Steel in 15% HCl Solution. Int. J. Electrochem. Sci. 2015, 10, 602–624. [Google Scholar]
- Fouda, A.S.; Diab, M.; El-Sonbati, A.; Hassan, S.A. Benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M phosphoric acid (H3PO4) solution. Afr. J. Pure Appl. Chem. 2013, 7, 67–78. [Google Scholar] [CrossRef]
- Salarvand, Z.; Amirnasr, M.; Talebian, M.; Raeissi, K.; Meghdadia, S. Enhanced corrosion resistance of mild steel in 1 M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros. Sci. 2017, 114, 133–145. [Google Scholar] [CrossRef]
- Hu, Z.; Meng, Y.; Ma, X.; Zhu, H.; Li, J.; Li, C.; Cao, D. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 2016, 112, 563–575. [Google Scholar] [CrossRef]
- Kobayashi, N.; Fukuda, T.; Ueno, K.; Ogino, H. Extremely Non-Planar Phthalocyanines with Saddle or Helical Conformation: Synthesis and Structural Characterizations. J. Am. Chem. Soc. 2001, 123, 10740–10741. [Google Scholar] [CrossRef]
- Dibetsoe, M.; Olasunkanmi, L.O.; Fayemi, O.E.; Yesudass, S.; Ramaganthan, B.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Some Phthalocyanine and Naphthalocyanine Derivatives as Corrosion Inhibitors for Aluminium in Acidic Medium: Experimental, Quantum Chemical Calculations, QSAR Studies and Synergistic Effect of Iodide Ions. Molecules 2015, 20, 15701–15734. [Google Scholar] [CrossRef] [Green Version]
- Aoki, I.V.; Guedes, I.G.; Maranhao, S.L. Copper phthalocyanine as corrosion inhibitor for ASTM A606-4 steel in 16% hydrochloric acid. J. Appl. Electrochem. 2002, 32, 915–919. [Google Scholar] [CrossRef]
- Zhao, P.; Liang, Q.; Li, Y. Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/L HCl. Appl. Surf. Sci. 2005, 252, 1596–1607. [Google Scholar] [CrossRef]
- Özdemir, O.K.; Aytaç, A.; Atilla, D.; Durmuş, M. Corrosion inhibition of aluminum by novel phthalocyanines in hydrochloric acid solution. J. Mater. Sci. 2011, 46, 752–758. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Alobaidy, A.H.M.; Mohamad, A.B.; Hoon, P.S. Novel Corrosion Inhibitor for Mild Steel in HCl. Materials 2014, 7, 662–672. [Google Scholar] [CrossRef]
- Zor, S. Sulfathiazole as potential corrosion inhibitor for copper in 0.1 M NaCl. Prot. Met. Phys. Chem. Surf. 2014, 50, 530–537. [Google Scholar] [CrossRef]
- Verma, C.; Obot, I.B.; Bahadura, I.; Sherif, E.S.M.; Ebenso, E.E. Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: Gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies. Appl. Surf. Sci. 2018, 457, 134–149. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Awad, K.A. Electrochemical Corrosion and Passivation Behaviour of Titanium and Its Ti-6Al-4V Alloy in Low and Highly Concentrated HBr Solutions. Int. J. Electrochem. Sci. 2011, 6, 6483–6502. [Google Scholar]
- Yan, Y.; Li, W.; Cai, L.; Hau, B. Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1 M HCl solution. Electrochim. Acta 2008, 53, 5953–5960. [Google Scholar] [CrossRef]
- Karthik, G.; Sundaravadivelu, M. Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug. Egypt. J. Pet. 2016, 25, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Mashuga, M.E.; Olasunkanmi, L.O.; Adekunle, A.S.; Yesudass, S.; Kabanda, M.M.; Ebenso, E.E. Adsorption, Thermodynamic and Quantum Chemical Studies of 1-hexyl-3-methylimidazolium Based Ionic Liquids as Corrosion Inhibitors for Mild Steel in HCl. Materials 2015, 8, 3607–3632. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.; Asghar, B.H.; Zaafarany, I.; Fouda, A.S. The Inhibition of Carbon Steel Corrosion in Hydrochloric Acid Solution using Some Phenolic Compounds. Int. J. Electrochem. Sci. 2012, 7, 282–304. [Google Scholar]
- Nnaji, N.J.N.; Okoye, C.O.B.; Obi-Egbedi, N.O.; Ezeokonkwo, M.A.; Ani, J.U. Spectroscopic Characterization of Red Onion Skin Tannin and It’s use as Alternative Aluminium Corrosion Inhibitor in Hydrochloric Acid Solutions. Int. J. Electrochem. Sci. 2013, 8, 1735–1758. [Google Scholar]
- Abd El Rehim, S.S.; Sayyah, S.M.; El-Deeb, M.M.; Kamal, S.M.; Azooz, R.E. Adsorption and corrosion inhibitive properties of P(2-aminobenzothiazole) on mild steel in hydrochloric acid media. Int. J. Ind. Chem. 2016, 7, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Emregul, K.C.; Hayvalı, M. Studies on the effect of a newly synthesized Schiff base compound from phenazone and vanillin on the corrosion of steel in 2 M HCl. Corros. Sci. 2006, 48, 797–812. [Google Scholar] [CrossRef]
- Saini, N.; Kumar, R.; Lgaz, H.; Salghi, R.; Chung, I.M.; Kumar, S.; Lata, S. Minified dose of urispas drug as better corrosion constraint for soft steel in sulphuric acid solution. J. Mol. Liq. 2018, 269, 371–380. [Google Scholar] [CrossRef]
- Gerengi, H.; Schaefer, K.; Sahin, H.I. Corrosion-inhibiting effect of Mimosa extract on brass-MM55 corrosion in 0.5 M H2SO4 acidic media. J. Ind. Eng. Chem. 2012, 18, 2204–2210. [Google Scholar] [CrossRef]
- Nnaji, N.J.; Obi-Egbedi, N.O.; Nnabugwu, M.A. Kinetics and Thermodynamics of Aluminium Corrosion Inhibitio shen by Anthocleista Djalonensis Leaf Extract in Hydrochloric Acid Solution. Int. J. Chem. Sci. 2012, 10, 182–194. [Google Scholar]
- Madkour, L.H.; Kaya, S.; Obot, I.B. Computational, Monte Carlo simulation and experimental studies of some arylazotriazoles (AATR) and their copper complexes in corrosion inhibition process. J. Mol. Liq. 2018, 260, 351–374. [Google Scholar] [CrossRef]
- Nnaji, N.J.N.; Obi-Egbedi, N.O.; Okoye, C.O.B. Cashew Nut Testa Tannin: Assessing its Effects on the Corrosion of Aluminium in HCl. Port. Electrochim. Acta 2014, 32, 157–182. [Google Scholar] [CrossRef]
- Aoun, S.A. On the corrosion inhibition of carbon steel in 1 M HCl with a pyridinium-ionic liquid: Chemical, thermodynamic, kinetic and electrochemical studies. RSC Adv. 2017, 7, 36688–36696. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Jodeh, S.; Hammouti, B. Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J. Mol. Liq. 2017, 225, 271–280. [Google Scholar] [CrossRef]
- Ahamada, I.; Prasad, R.; Quraishia, M.A. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472–1481. [Google Scholar] [CrossRef]
- Meng, Y.; Ning, W.; Xu, B.; Yang, W.; Zhang, K.; Chen, Y.; Li, L.; Liu, X.; Zhenga, J.; Zhang, Y. Inhibition of mild steel corrosion in hydrochloric acid using two novel pyridine Schiff base derivatives: A comparative study of experimental and theoretical results. RSC Adv. 2017, 7, 43014–43029. [Google Scholar] [CrossRef]
- Li, X.; Denga, S.; Li, N.; Xiec, X. Inhibition effect of bamboo leaves extract on cold rolled steel in Cl3CCOOH solution. J. Mater. Res. Technol. 2017, 6, 158–170. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, M.; Zhenga, J.; Castaneda, H. Corrosion inhibition of mild steel by an imidazolium ionic liquid compound: The effect of pH and surface pre-corrosion. RSC Adv. 2015, 5, 95160–95170. [Google Scholar] [CrossRef]
- Hachelef, H.; Benmoussat, A.; Khelifa, A.; Athmani, D.; Bouchareb, D. Study of corrosion inhibition by Electrochemical Impedance Spectroscopy method of 5083 aluminum alloy in 1 M HCl solution containing propolis extract. J. Mater. Environ. Sci. 2016, 7, 1751–1758. [Google Scholar]
- Halambek, J.; Berković, K. Inhibitive Action of Anethum graveolens L. oil on Aluminium Corrosion in Acidic Media. Int. J. Electrochem. Sci. 2012, 7, 8356–8368. [Google Scholar]
- Rajendran, S.; Jeyasundari, J.; Usha, P.; Selvi, J.A.; Narayanasamy, B.; Regis, A.P.P.; Rengan, P. Corrosion Behaviour of Aluminium in the Presence of an Aqueous Extract of Hibiscus Rosa-sinensis. Port. Electrochim. Acta 2009, 27, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Ziminov, A.V.; Pudova, D.I.; Kolganova, A.I.; Stretovich, M.A.; Furman, M.A.; Ramsh, S.M. Synthesis of 4-(4-Hydrazinylphenoxy)phthalonitrile and Phthalonitriles on Its Basis Containing N-Heterocycles. Macroheterocycles 2015, 8, 26–31. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Ravichandran, J. Aqueous extract of leaves of Morinda tinctoria as a corrosion inhibitor for aluminum in sulphuric acid medium. J. Adhes. Sci. Technol. 2015, 29, 1465–1482. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, R.; Liu, H.; Liu, Y.; Li, S.; Niu, L. Electrochemical and surface analysis studies of 2-(quinolin-2-yl)quinazolin-4(3H)-one as corrosion inhibitor for Q235 steel in hydrochloric acid. J. Mol. Liq. 2016, 222, 671–679. [Google Scholar] [CrossRef]
- Prabhu, D.; Rao, P. Garcinia indica as an Environmentally Safe Corrosion Inhibitor for Aluminium in 0.5 M Phosphoric Acid. Int. J. Corros. 2013, 2013, 945143. [Google Scholar] [CrossRef]
- Omaka, O.N.; Ekennia, A.C.; Nwaji, N.N.; Onwudiwe, D.C. Nickel(II) and copper(II) complexes of 2,2′-bibenzo[d] thiazole: Synthesis, characterisation and biological studies. Appl. Organomet. Chem. 2018, 32, e4241. [Google Scholar] [CrossRef]
- Sekhosana, K.E.; Nyokong, T. Optical limiting response of multi-walled carbon nanotubephthalocyanine nanocomposite in solution and when in poly (acrylic acid). J. Mol. Struct. 2016, 1117, 140–146. [Google Scholar] [CrossRef]
- Oluwole, D.O.; Nyokong, T. Photophysicochemical behaviour of metallophthalocyanines when doped onto silica nanoparticles. Dyes Pigment. 2017, 136, 262–272. [Google Scholar] [CrossRef]
- Sen, P.; Yildiz, S.Z.; Erdoğmuş, A.; Dege, N.; Atalay, Y. Aldehyde Substituted Phthalocyanines: Synthesis, Characterization and Investigation of Photophysical and Photochemical Properties. J. Fluoresc. 2016, 26, 1521–1534. [Google Scholar] [CrossRef]
- Kammers, A.D.; Ngam, J.W.; Langdon, T.G.; Daly, S. The effect of microstructure heterogeneity on the microscale deformation of ultrafine-grained aluminum. J. Mater. Res. 2014, 29, 1664–1674. [Google Scholar] [CrossRef]
- Rosa, D.M.; Spinelli, J.E.; Os´orio, W.R.; Garcia, A. Effects of cell size and macrosegregation on the corrosion behavior of a dilute Pb–Sb alloy. J. Power Sources 2006, 162, 696–705. [Google Scholar] [CrossRef]
- Osório, W.R.; Cheung, N.; Spinelli, J.E.; Goulart, P.R.; Garcia, A. The effects of a eutectic modifier on microstructure and surface corrosion behavior of Al-Si hypoeutectic alloys. J. Solid State Electrochem. 2007, 11, 1421–1427. [Google Scholar] [CrossRef]
- Handy, A.; El-Gendy, N.S. Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt. J. Pet. 2013, 22, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Leetmaa, K.; Gomez, M.A.; Becze, L.; Guo, F.; Demopoulos, G.P. Comparative molecular characterization of aluminum hydroxy-gels derived from chloride and sulphate salts. J. Chem. Technol. Biotechnol. 2014, 89, 206–213. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Gregory, M.R.; Barton, R. Bayerite, nordstrandite, gibbsite, brucite, and pseudo boehmite in discharged caustic waste from Campbell Island, southwest Pacific. Clays Clay Miner. 1991, 39, 103–107. [Google Scholar] [CrossRef]
- Kozawa, T.; Naito, M. Mechanically induced formation of metastable v- and j-Al2O3 from boehmite. Adv. Powder Technol. 2016, 27, 935–939. [Google Scholar] [CrossRef]
- Karami, C.; Abdollahifar, M.; Jahani, F.; Farrokhi, A.; Taher, M.A. The preparation and characterization of flower-like boemite nanoparticles-SA: A new and reusable nanocatalyst for the synthesis of 2-aryl-1H-benzimidazoles. Inorg. Nano-Met. Chem. 2017, 47, 626–631. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Zamani, M.R.; Beiygie, E.; Nekouei, H. Synthesis of micro-mesopores flower-like γ-Al2O3 nano-architectures. J. Serb. Chem. Soc. 2014, 79, 1007–1017. [Google Scholar] [CrossRef]
- Mahendiran, C.; Ganesan, R.; Gedanken, A. Sonoelectrochemical synthesis of metallic aluminium nanoparticles. Eur. J. Inorg. Chem. 2009, 2050–2053. [Google Scholar] [CrossRef]
- Lei, X.F.; Ma, J.X. Synthesis and electrochemical performance of aluminium based composites. J. Braz. Chem. Soc. 2010, 21, 209–213. [Google Scholar] [CrossRef]
- Gottapu, S.; Padhi, S.K.; Krishna, M.G.; Muralidharan, K. Poly(vinylpyrrolidone) stabilized aluminium nanoparticles obtained by the reaction of SiCl4 with LiAlH4. New J. Chem. 2015, 39, 5203–5207. [Google Scholar] [CrossRef]
- Azooz, R.E. EDTA as a corrosion inhibitor for Al in 0.5 M HCl: Adsorption, thermodynamic and theoretical study. J. Electrochem. Sci. Eng. 2016, 6, 235–251. [Google Scholar] [CrossRef]
- Kumar, T.V.; Makangara, J.; Laxmikanth, C.; Babu, N.S. Computational Studies for Inhibitory Action of 2-Mercapto-1-Methylimidazole Tautomers on Steel Using of Density Functional Theory Method (DFT). Int. J. Comput. Theoret. Chem. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Wazzan, N.A.; Mahgoub, F.M. DFT Calculations for Corrosion Inhibition of Ferrous Alloys by Pyrazolopyrimidine Derivatives. Open J. Phys. Chem. 2014, 4, 6–14. [Google Scholar] [CrossRef]
- Mohamed Abdelahi, M.M.; Elmsellem, H.; Benchidmi, M.; Sebbar, N.K.; Belghiti, M.A.; El Ouasif, L.; Jilalat, A.E.; Kadmi, Y.; Essassi, E.M. A DFT and Molecular Dynamics Study on Inhibitory Action of indazole Derivative on Corrosion of Mild Steel. J. Mater. Environ. Sci. 2017, 8, 1860–1876. [Google Scholar]
- Anusuya, N.; Sounthari, P.; Saranya, J.; Parameswari, K.; Chitra, S. Corrosion inhibition effect of hydroxy pyrazoline derivatives on mild steel in sulphuric acid solution together with Quantum chemical studies. J. Mater. Environ. Sci. 2015, 6, 1606–1623. [Google Scholar]
- Taghavikish, M.; Dutta, N.K.; Choudhury, N.R. Emerging Corrosion Inhibitors for Interfacial Coating. Coatings 2017, 7, 217. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.O.; Obot, I.B. Xanthione: A new and effective corrosion inhibitor for mild steel in sulphuric acid solution. Arab. J. Chem. 2013, 6, 211–223. [Google Scholar] [CrossRef] [Green Version]
- El-Etre, A.Y. Inhibition of aluminum corrosion using Opuntia extract. Corros. Sci. 2003, 45, 2485–2495. [Google Scholar] [CrossRef]
- Okafor, P.C.; Ikpi, M.E.; Uwah, I.E.; Ebenso, E.E.; Ekpe, U.J.; Umoren, S.A. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- Nwaji, N.; Bankole, O.M.; Britton, J.; Nyokong, T. Photophysical and nonlinear optical study of benzothiazole substituted phthalocyanines in solution and thin films. J. Porphyr. Phthalocyanines 2017, 21, 263–272. [Google Scholar] [CrossRef]
- Mishra, A.K.; Balasubramanian, R. Corrosion inhibition of aluminum alloy AA 2014 by rare earth chlorides. Corros. Sci. 2007, 49, 1027–1044. [Google Scholar] [CrossRef]
- Ghareba, S.; Omanovic, S. The effect of electrolyte flow on the performance of 12-aminododecanoic acid as a carbon steel corrosion inhibitor in CO2-saturated hydrochloric acid. Corros. Sci. 2011, 53, 3805–3812. [Google Scholar] [CrossRef]
- Ghareba, S.; Omanovic, S. 12-Aminododecanoic acid as a corrosion inhibitor for carbon steel. Electrochim. Acta 2011, 56, 3890–3898. [Google Scholar] [CrossRef]
- Shirazi, Z.; Keshavarz, M.H.; Esmaeilpour, K.; Pakniya, T. A novel and simple method for the prediction of corrosion inhibition efficiency without using complex computer codes. Z. Anorg. Allg. Chem. 2017, 643, 2149–2157. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, G.; Scalmani, J.R.; Barone, V.; Mennucci, B.; Petersson, H.; et al. Gaussian 09, Revision A. 1; Gaussian Inc.: Wallingford, CO, USA, 2009. [Google Scholar]
Sample Availability: Not available. |














| Concentration (µM) | −Eicorr (mA cm−2) | −βa (mV dec−1) | IE% | ||
|---|---|---|---|---|---|
| 0 | 672.0 ± 4.0 | 8.68 ± 0.44 | 493.8 ± 3.1 | 91.8 ± 1.6 | - |
| BTThio | |||||
| 2 | 652.0 ± 8.0 | 4.64 ± 0.8 | 362.3 ± 4.8 | 231.1 ± 7.1 | 46.9 ± 6.5 |
| 4 | 696.0 ± 6.0 | 4.13 ± 0.24 | 369.8 ± 5.5 | 239.7 ±1.3 | 52.5 ± 0.35 |
| 6 | 688.0 ± 16.0 | 3.48 ± 0.66 | 201.3 ± 4.3 | 129.1 ± 2.8 | 59.9 ± 1.8 |
| 8 | 700.0 ± 6.0 | 3.04 ± 0.11 | 279.4 ± 3.7 | 219.4 ± 6.3 | 64.9 ± 3.8 |
| 10 | 676.0 ± 14.0 | 2.54 ± 0.33 | 278.1 ± 2.1 | 236.8 ± 5.5 | 70.8 ± 1.3 |
| ClGaBTThioPc | |||||
| 2 | 680.0 ± 4.0 | 3.50 ± 0.25 | 267.5 ± 3.8 | 131.6 ± 3.6 | 59.7 ± 0.8 |
| 4 | 692.0 ± 3.0 | 2.65 ± 0.23 | 139.5 ± 2.9 | 79.1 ± 2.8 | 69.5 ± 0.9 |
| 6 | 692.0 ± 4.0 | 2.28 ± 0.10 | 94.8 ± 4.3 | 71.7 ± 7.8 | 73.8 ± 0.1 |
| 8 | 732.0 ± 3.0 | 1.65 ± 0.05 | 112.4 ± 1.4 | 172.2 ± 1.4 | 81.0 ± 0.2 |
| 10 | 664.0 ± 4.0 | 1.72 ± 0.05 | 157.8 ± 4.2 | 69.9 ± 0.5 | 80.2 ± 0.6 |
| Isotherm | Equilibrium Constant (K) (M−1) b | Free Energy of Adsorption (kJmol−1) | Adsorption Constants | Fit to Equation | χ2 |
|---|---|---|---|---|---|
| BTThio | |||||
| Langmuir | 5.0 × 105 | −42.9 | - | No | 10.9779 |
| Freundlich | 13.1 | −16.5 | n = 3.9 | Yes | 0.1679 |
| Temkin | 1.07 × 107 | −50.6 | f = 6.8 | Yes | 0.3001 |
| El-Awady | 2.5 × 103 | −29.6 | YEl = 0.6 | Yes | 0.3677 |
| Frumkin | 3.4 × 10−7 M a | +27.2 | α = 1.6 | No | 15.5670 |
| ClGaBTThioPc | |||||
| Langmuir | 1.0 × 106 | −44.6 | - | No | 5.7350 |
| Freundlich | 7.7 | −15.2 | n = 5.1 | Yes | 0.1134 |
| Temkin | 4.1 × 107 | −53.9 | f = 7.4 | Yes | 0.1130 |
| El-Awady | 1.0 × 104 | −33.1 | YEl = 0.7 | Yes | 0.1136 |
| Frumkin | 1.5 × 10−7 M a | +29.3 | α = 2.5 | No | 10.0029 |
| Concentration (µM) | n | Rt (Ωcm2) | IE% |
|---|---|---|---|
| 0 | 0.939 ± 0.005 | 26.6 ± 0.16 | - |
| BTThio | |||
| 2 | 0.924 ± 0.000 | 48.81 ± 0.65 | 45.5 ± 0.4 |
| 4 | 0.928 ± 0.100 | 54.22 ± 1.76 | 50.9 ± 1.3 |
| 6 | 0.927 ± 0.000 | 61.15 ± 0.37 | 58.6 ± 2.1 |
| 8 | 0.934 ± 0.000 | 72.91 ± 2.10 | 63.5 ± 0.8 |
| 10 | 0.922 ± 0.000 | 85.09 ± 1.83 | 68.7 ± 0.5 |
| ClGaBTThioPc | |||
| 2 | 0.924 ± 0.000 | 59.52 ± 0.58 | 55.3 ± 0.7 |
| 4 | 0.940 ± 0.016 | 85.24 ± 4.39 | 68.7 ± 1.8 |
| 6 | 0.940 ± 0.000 | 114.92 ± 16.26 | 76.4 ± 3.2 |
| 8 | 0.901 ± 0.080 | 157.40 ± 0.36 | 83.1 ± 0.14 |
| 10 | 0.922 ± 0.040 | 140.87 ± 3.78 | 81.1 ± 0.62 |
| Inhibitor Molecule | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | η (eV) | χ (eV) | ΔN | δ (eV) |
|---|---|---|---|---|---|---|---|
| ClGaBTThioPc | −6.12 | −3.66 | 2.46 | 1.23 | 4.89 | −0.83 | 0.81 |
| BTThio | −8.14 | −2.56 | 5.59 | 2.79 | 5.35 | −1.06 | 0.358 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nnaji, N.; Nwaji, N.; Mack, J.; Nyokong, T. Corrosion Resistance of Aluminum against Acid Activation: Impact of Benzothiazole-Substituted Gallium Phthalocyanine. Molecules 2019, 24, 207. https://doi.org/10.3390/molecules24010207
Nnaji N, Nwaji N, Mack J, Nyokong T. Corrosion Resistance of Aluminum against Acid Activation: Impact of Benzothiazole-Substituted Gallium Phthalocyanine. Molecules. 2019; 24(1):207. https://doi.org/10.3390/molecules24010207
Chicago/Turabian StyleNnaji, Nnaemeka, Njemuwa Nwaji, John Mack, and Tebello Nyokong. 2019. "Corrosion Resistance of Aluminum against Acid Activation: Impact of Benzothiazole-Substituted Gallium Phthalocyanine" Molecules 24, no. 1: 207. https://doi.org/10.3390/molecules24010207
APA StyleNnaji, N., Nwaji, N., Mack, J., & Nyokong, T. (2019). Corrosion Resistance of Aluminum against Acid Activation: Impact of Benzothiazole-Substituted Gallium Phthalocyanine. Molecules, 24(1), 207. https://doi.org/10.3390/molecules24010207