Quantification of the Organic Acids in Hawthorn Wine: A Comparison of Two HPLC Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Analysis of Hawthorn Wine
2.2. Identification of Organic Acids in Hawthorn Wine with the Enzymatic Method
2.3. Validation of the two HPLC Methods
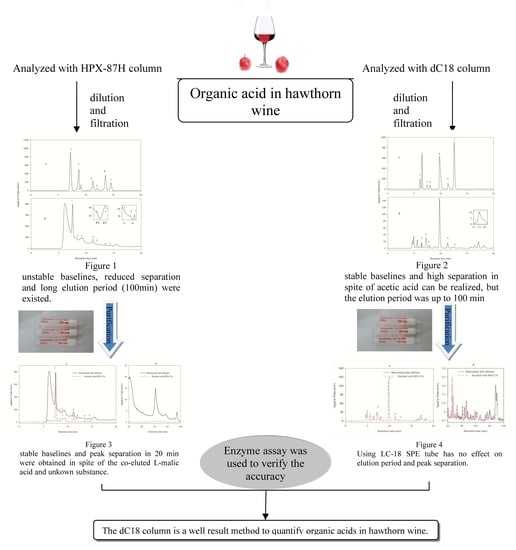
2.4. Analysis of Organic Acids with HPLC Method 1
2.5. Analysis of Organic Acids with HPLC Method 2
2.6. Pretreatment of the Samples with LC-18 SPE Tube
2.6.1. Recovery of Organic Acids Treated with LC-18 SPE Tube
2.6.2. Effect of LC-18 SPE Tube Pretreatment on the Analysis of HPLC Method 1
2.6.3. Effect of LC-18 SPE Tube Pretreatment on the Analysis of HPLC Method 2
2.7. Quantification of Arganic Acids with Two HPLC Methods
3. Materials and Methods
3.1. Materials and Reagents
3.2. Technological Process of Hawthorn Wine Making
3.3. Chemical Analysis
3.4. Identification of Organic Acids with the Enzymatic Method
3.5. Quantification of Organic Acids with HPLC Method 1
3.5.1. Pretreatment of Samples
3.5.2. Chromatographic Condition
3.5.3. Quantification of Organic Acids
3.6. Quantification of Organic Acids with HPLC Method 2
3.6.1. Pretreatment of Samples
3.6.2. Chromatographic Condition
3.6.3. Quantification of Organic Acids
3.7. Pretreatment with LC-18 SPE Tube
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rigelsky, J.M.; Sweet, B.V. Hawthorn wine: Pharmacology and therapeutic uses. Am. J. Health Syst. Ph. 2002, 59, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Ho, W.K.K.; Huang, Y.; Chen, Z.Y. Hypocholesterolemic activity of hawthorn wine fruit is mediated by regulation of cholesterol-7alpha-hydroxylase and acyl COA: Cholesterol acyltransferase. Food Res. Int. 2002, 35, 885–891. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Voiselle, G.; Fruchart, J.C.; Duriez, P.; Teissier, E.; Bailleul, F.; Vasseur, J.; Trotin, F. Hawthorn wine extracts inhibit LDL oxidation. Pharmazie 2003, 58, 577–581. [Google Scholar] [PubMed]
- Tassell, M.C.; Rosari, K.; Deirdre, G.; Mary, L.; Ambrose, F. Hawthorn wine (Crataegus spp.) in the treatment of cardiovascular disease. Pharm. Rev. 2010, 4, 32–41. [Google Scholar]
- Zhu, R.G.; Sun, Y.D.; Li, T.P.; Chen, G.; Peng, X.; Duan, W.B.; Zheng, Z.Z.; Shi, S.L.; Xu, J.G.; Liu, Y.H.; et al. Comparative effects of hawthorn wine (Crataegus pinnatifida, bunge) pectin and pectin hydrolyzates on the cholesterol homeostasis of hamsters fed high-cholesterol diets. Chem. Biol. Interact. 2015, 238, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Aierken, A.; Buchholz, T.; Chen, C.; Zhang, X.; Melzig, M.F. Hypoglycemic effect of hawthorn wine in type II diabetes mellitus rat model. J. Sci. Food Agr. 2017, 97, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Z.; Kallio, H.; Lü, D.G.; Zhou, C.S.; Ou, S.Y.; Yang, B.R. Acids, sugars, and sugar alcohols in Chinese hawthorn wine (Crataegus spp.) fruits. J. Agr. Food Chem. 2010, 58, 1012–1019. [Google Scholar] [CrossRef]
- Silva, F.L.D.N.; Schmidt, E.M.; Messias, C.L.; Eberlin, M.N. Quantitation of organic acids in wine and grapes by direct infusion electrospray ionization mass spectrometry. Anal. Methods UK 2014, 7, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.T.; Du, J.H.; He, G.F. Influence of fruit yeasts on quality and antioxidant capability of hawthorn wine wine. Liquor making 2012, 39, 52–56. [Google Scholar]
- He, G.F.; Sui, J.L.; Du, J.H.; Lin, J. Characteristics and antioxidant capacities of five hawthorn wine wines fermented by different wine yeasts. J. Inst. Brewing 2013, 119, 321–327. [Google Scholar] [CrossRef]
- Liu, S.W.; Zhang, X.; You, L.; Guo, Z.Y.; Chang, X.D. Changes in anthocyanin profile, color, and antioxidant capacity of hawthorn wine wine (Crataegus pinnatifida) during storage by pretreatments. LWT Food Sci. Technol. 2018, 95, 179–186. [Google Scholar] [CrossRef]
- Liu, S.W.; Chang, X.D.; Liu, X.F.; Shen, Z.E. Effects of pretreatments on anthocyanin composition, phenolics contents and antioxidant capacities during fermentation of hawthorn wine (Crataegus pinnatifida) drink. Food Chem. 2016, 212, 87–95. [Google Scholar] [CrossRef]
- Dong, W.J.; Du, J.H.; Fu, Y.Z. Research of hawthorn wine wine with reduced methanol content. Liquor Making 2015, 42, 40–45. [Google Scholar]
- Anthon, G.E.; Lestrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agr. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]
- Velterop, J.S.; Vos, F. A rapid and inexpensive microplate assay for the enzymatic determination of glucose, fructose, sucrose, l-malate and citrate in tomato (Lycopersicon esculentum) extracts and in orange juice. Phytochem. Anal. 2001, 12, 299–304. [Google Scholar] [CrossRef]
- Vermeir, S.; Nicolai, B.; Jans, K.; Maes, G.; Lammertyn, J. High-throughput microplate enzymatic assays for fast sugar and acid quantification in apple and tomato. J. Agr. Food Chem. 2007, 55, 3240–3248. [Google Scholar] [CrossRef]
- Arraezroman, D.; Fernandezsanchez, J.F.; Cortaceroramirez, S.; Seguracarretero, A.; Fernandezgutierrez, A. A simple light-emitted diode-induced fluorescence detector using optical fibers and a charged coupled device for direct and indirect capillary electrophoresis methods. Electrophoresis 2006, 27, 1776–1783. [Google Scholar] [CrossRef]
- Peres, R.G.; Moraes, E.P.; Micke, G.A.; Tonin, F.G.; Tavares, M.F.M.; Rodriguez-Amaya, D.B. Rapid method for the determination of organic acids in wine by capillary electrophoresis with indirect UV detection. Food Control. 2009, 20, 548–552. [Google Scholar] [CrossRef]
- Jham, G.N.; Fernandes, S.A.; Garcia, C.F.; Da, S.A. Comparison of GC and HPLC for the quantification of organic acids in coffee. Phytochem. Anal. 2002, 13, 99–104. [Google Scholar] [CrossRef]
- Stelios, K.; Paraskeri, S.; Theophiles, M. Comparison of the characteristics of set type yoghurt made from ovine milk of different fat content. Int. J. Food Sci. Tech. 2007, 42, 1019–1028. [Google Scholar]
- Tanaka, K.; Mori, M.; Xu, Q.; Helaleh, M.I.H.; Ikedo, M.; Taoda, H.; Hu, W.; Hasebe, K.; Fritz, J.S.; Haddad, P.R. Ion-exclusion chromatography with conductimetric detection of aliphatic carboxylic acids on a weakly acidic cation-exchange resin by elution with benzoic acid-β-cyclodextrin. J. Chromatogr. A. 2003, 997, 127–132. [Google Scholar]
- Tormo, M.; Izco, J.M. Alternative reversed-phase high-performance liquid chromatography method to analyse organic acids in dairy products. J. Chromatogr. A. 2004, 1033, 305–310. [Google Scholar] [CrossRef]
- Scherer, R.; Rybka, A.C.P.; Ballus, C.A.; Meinhart, A.D.; Filho, J.T.; Godoy, H.T. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012, 135, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Coelho, E.M.; Padilha, C.V.; Miskinis, G.A.; De Sá, A.G.B.; Pereira, G.E.; Azevedo, L.; Lima, M.D. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from Northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- Pereira, V.; Câmara, J.S.; Cacho, J.; Marques, J.C. HPLC-DAD methodology for the quantification of organic acids, furans and polyphenols by direct injection of wine samples. J. Sep. Sci. 2010, 33, 1204–1215. [Google Scholar] [CrossRef] [Green Version]
- Kadakal, C.; Nas, S. Effect of activated charcoal on patulin, fumaric acid and some other properties of apple juice. Die Nahrung 2002, 46, 31–33. [Google Scholar] [CrossRef]
- Gökmen, V.; Acar, J. Fumaric acid in apple juice: A potential indicator of microbial spoilage of apples used as raw material. Food Addit Contam. 2004, 21, 626–631. [Google Scholar] [CrossRef]
- Shapiro, F.; Silanikove, N. Rapid and accurate determination of malate, citrate, pyruvate and oxaloacetate by enzymatic reactions coupled to formation of a fluorochromophore: Application in colorful juices and fermentable food (yogurt, wine) analysis. Food Chem. 2011, 129, 608–613. [Google Scholar] [CrossRef]
- Konieczka, P. The role of and the place of method validation in the quality assurance and quality control (QA/QC) system. Crit. Rev. Anal. Chem. 2007, 37, 173–190. [Google Scholar] [CrossRef]
- Gao, Y.; Ierapetritou, M.G.; Muzzio, F.J. Determination of the confidence interval of the relative standard deviation using convolution. J. Pharm. Innov. 2013, 8, 72–82. [Google Scholar] [CrossRef]
- Agius, C.; Tucher, S.V.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. Methodsx 2018, 5, 537–550. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Cristina, G.M.; Luh, B.S. HPLC analysis of organic acids and sugars in tomato juice. J. Food Sci. 2010, 51, 571–573. [Google Scholar]
- Marconi, O.; Floridi, S.; Montanari, L. Organic acids profile in tomato juice by HPLC with UV detection. J. Food Qual. 2007, 30, 253–266. [Google Scholar] [CrossRef]
- Rozhon, W.; Petutschnig, E.; Wrzaczek, M.; Jonak, C. Quantification of free and total salicylic acid in plants by solid-phase extraction and isocratic high-performance anion-exchange chromatography. Anal. Bioanal. Chem. 2005, 382, 1620–1627. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| Samples | Alcohol (%, v/v) | Reducing Sugar (g/L) | Sugar-Free Extract (g/L) | Total Acidity (g/L) | pH |
|---|---|---|---|---|---|
| 1 | 14.69 ± 0.01 b | 5.60 ± 0.04 a | 37.12 ± 0.06 a | 14.51 ± 0.00 b,c | 3.22 ± 0.01 c |
| 2 | 14.83 ± 0.01 b | 5.53 ± 0.07 a | 36.77 ± 0.38 a,b | 13.99 ± 0.00 c,d | 3.21 ± 0.00 c |
| 3 | 15.39 ± 0.01 a | 4.28 ± 0.00 c | 32.35 ± 0.18 e | 13.15 ± 0.09 e | 3.28 ± 0.01 a |
| 4 | 15.34 ± 0.00 a | 4.00 ± 0.07 c | 35.13 ± 0.33 c,d | 14.19 ± 0.04 c | 3.08 ± 0.00 f |
| 5 | 14.89 ± 0.01 b | 5.10 ± 0.07 b | 34.78 ± 0.03 d | 13.51 ± 0.02 d,e | 3.25 ± 0.00 b |
| 6 | 15.42 ± 0.20 a | 4.11 ± 0.09 c | 37.19 ± 0.47 a | 15.16 ± 0.04 a | 3.25 ± 0.00 b |
| 7 | 14.95 ± 0.01 b | 5.15 ± 0.07 b | 36.36 ± 0.01 a,b,c | 14.97 ± 0.00 a,b | 3.09 ± 0.00 e,f |
| 8 | 15.45 ± 0.07 a | 5.75 ± 0.11 a | 35.56 ± 0.62 b,c,d | 15.07 ± 0.05 a | 3.14 ± 0.01 d |
| 9 | 14.95 ± 0.01 b | 3.51 ± 0.12 d | 36.83 ± 0.35 a,b | 13.40 ± 0.11 e | 3.28 ± 0.01 a |
| 10 | 14.84 ± 0.00 b | 5.18 ± 0.04 b | 37.45 ± 0.30 a | 15.14 ± 0.03 a | 3.10 ± 0.00 e |
| Samples | Methods | Citric Acid (mg/L) | Succinic Acid (mg/L) | l-malic Acid (mg/L) | Acetic Acid (mg/L) | Lactic Acid (mg/L) | Pyruvic Acid (mg/L) | Fumaric Acid (mg/L) | TOA (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | HPLC 1 a | 9587.5 ± 150.3 a | 2830.1 ± 62.2 a | 772.1 ± 50.2 a | 993.8 ± 158.2 a | 88.9 ± 6.2 b | 1.4 ± 0.2 b | 1.1 ± 0.1 c | 14.3 ± 0.4 |
| HPLC 2 b | 9928.7 ± 274.2 a | 1611.2 ± 91.8 b | 835.4 ± 1.7 a | 740.5 ± 10.0 a | 940.2 ± 6.2 a | 7.0 ± 0.1 a | 2.5 ± 0.1 b | 14.1 ± 0.4 | |
| EM c | 9372.1 ± 214.2 a | 1522.8 ± 47.2 b | 812.3 ± 2.8 a | 679.4 ± 7.2 a | 95.2 ± 2.3 c | 7.4 ± 0.3 a | 3.4 ± 0.6 a | 12.5 ± 0.3 | |
| 2 | HPLC 1 | 9315.4 ± 133.0 a,b | 2919.8 ± 1.8 a | 734.4 ± 7.5 a | 1073.9 ± 2.1 a | 86.5 ± 9.0 b | 0.9 ± 0.0 b | 1.1 ± 0.0 c | 14.1 ± 0.2 |
| HPLC 2 | 9785.0 ± 246.7 a | 1771.9 ± 61.2 b | 762.7 ± 9.8 a | 736.5 ± 18.4 b | 910.7 ± 12.1 a | 6.0 ± 0.2 a | 2.8 ± 0.1 b | 14.0 ± 0.3 | |
| EM | 8896.9 ± 192.0 b | 1571.6 ± 29.0 c | 745.0 ± 21.3 a | 643.9 ± 17.9 c | 103.0 ± 1.1 b | 6.2 ± 0.5 a | 3.7 ± 0.0 a | 12.0 ± 0.3 | |
| 3 | HPLC 1 | 8864.9 ± 282.9 a | 3110.3 ± 155.3 a | 484.1 ± 123.9 a | 892.1 ± 150.6 a | 102.3 ± 26.4 b | 1.3 ± 0.1 c | 1.2 ± 0.1 c | 13.5 ± 0.7 |
| HPLC 2 | 8744.5 ± 62.0 b | 1797.1 ± 54.7 b | 591.6 ± 2.9 a | 662.3 ± 12.9 a | 1114.2 ± 13.0 a | 7.0 ± 0.2 a | 2.6 ± 0.0 b | 12.9 ± 0.1 | |
| EM | 7967.6 ± 88.7 c | 1694.9 ± 14.5 b | 603.2 ± 14.2 a | 598.3 ± 10.8 a | 120.8 ± 2.7 c | 5.6 ± 0.5 b | 3.3 ± 0.0 a | 11.0 ± 0.1 | |
| 4 | HPLC 1 | 10230.0 ± 257.4 a | 2993.0 ± 23.1 a | 782.5 ± 33.4 b | 720.6 ± 56.4 a | 94.6 ± 9.5 b | 2.6 ± 0.0 c | 1.6 ± 0.2 b | 14.8 ± 0.4 |
| HPLC 2 | 9104.4 ± 80.8 b | 1753.8 ± 28.8 b | 681.5 ± 1.5 c | 665.2 ± 5.0 b | 1127.6 ± 5.1 a | 8.9 ± 0.4 a | 2.5 ± 0.0 a | 13.3 ± 0.1 | |
| EM | 9821.3 ± 132.6 a | 1664.1 ± 14.5 c | 983.2 ± 31.3 a | 694.6 ± 7.2 a | 106.1 ± 1.8 c | 4.9 ± 0.7 b | 2.5 ± 0.1 a | 13.3 ± 0.2 | |
| 5 | HPLC 1 | 9242.9 ± 130.8 a | 3092.1 ± 54.8 a | 742.3 ± 62.5 a | 959.1 ± 163.5 a | 87.6 ± 14.6 b | 1.1 ± 0.1 b | 1.1 ± 0.1 c | 14.1 ± 0.4 |
| HPLC 2 | 9196.6 ± 4.4 a | 1741.6 ± 30.3 b | 716.4 ± 6.2 a | 694.3 ± 3.3 a | 950.7 ± 5.3 a | 6.5 ± 0.2 a | 2.8 ± 0.1 b | 13.3 ± 0.0 | |
| EM | 8881.3 ± 95.9 a | 1640.9 ± 32.7 b | 716.8 ± 1.4 a | 616.0 ± 21.5 a | 89.9 ± 1.6 c | 6.9 ± 1.3 a | 3.3 ± 0.3 a | 12.0 ± 0.2 | |
| 6 | HPLC 1 | 9493.9 ± 6.1 a | 3261.9 ± 5.0 a | 501.7 ± 54.8 a | 982.6 ± 24.1 a | 86.9 ± 14.6 b | 2.0 ± 0.1 c | 1.7 ± 0.0 c | 14.3 ± 0.1 |
| HPLC 2 | 9321.1 ± 286.4 a | 1668.6 ± 111.3 b | 733.1 ± 5.2 a | 683.2 ± 2.0 b | 1131.2 ± 3.4 a | 7.5 ± 0.2 a | 2.7 ± 0.1 b | 13.5 ± 0.4 | |
| EM | 8855.2 ± 0.0 a | 1607.6 ± 58.1 b | 701.7 ± 93.8 a | 603.3 ± 17.9 c | 102.4 ± 1.6 c | 4.8 ± 0.1 b | 3.5 ± 0.2 a | 11.9 ± 0.2 | |
| 7 | HPLC 1 | 9111.0 ± 472.2 a | 2613.4 ± 156.3 a | 771.5 ± 11.3 a | 866.1 ± 162.6 a | 66.0 ± 6.1 b | 0.9 ± 0.3 c | 1.0 ± 0.2 c | 13.4 ± 0.8 |
| HPLC 2 | 9709.1 ± 158.4 a | 1573.4 ± 36.8 b | 877.7 ± 2.8 a,b | 761.1 ± 15.6 a | 795.0 ± 2.6 a | 7.1 ± 0.3 a | 2.4 ± 0.1 b | 13.7 ± 0.2 | |
| EM | 9090.0 ± 0.0 a | 1468.9 ± 14.5 b | 878.7 ± 42.6 b | 723.7 ± 19.7 a | 86.2 ± 6.8 c | 5.3 ± 0.7 b | 2.8 ± 0.0 a | 12.3 ± 0.1 | |
| 8 | HPLC 1 | 9753.4 ± 140.8 b | 2165.8 ± 55.9 a | 701.3 ± 20.2 a | 1111.9 ± 127.9 a | 61.9 ± 0.7 b | 0.6 ± 0.1 b | 1.0 ± 0.1 c | 13.8 ± 0.3 |
| HPLC 2 | 10156.6 ± 113.3 a | 1456.0 ± 30.6 b | 756.3 ± 0.3 a | 908.1 ± 17.7 b | 740.1 ± 3.8 a | 5.9 ± 0.2 a | 2.5 ± 0.0 b | 14.0 ± 0.2 | |
| EM | 9173.7 ± 44.3 c | 1327.7 ± 3.6 b | 754.0 ± 37.0 a | 818.8 ± 0.0 b | 72.7 ± 0.5 c | 6.1 ± 0.4 a | 3.3 ± 0.4 a | 12.2 ± 0.1 | |
| 9 | HPLC 1 | 9069.4 ± 61.5 a | 3141.3 ± 66.8 a | 576.5 ± 137.1 a | 760.8 ± 106.7 a | 105.9 ± 14.4 b | 2.5 ± 0.2 c | 1.3 ± 0.1 c | 13.7 ± 0.4 |
| HPLC 2 | 8843.8 ± 61.9 a | 1857.4 ± 47.6 b | 659.2 ± 0.1 a | 616.8 ± 7.8 b | 1140.2 ± 1.9 a | 8.8 ± 0.0 a | 2.8 ± 0.0 b | 13.1 ± 0.1 | |
| EM | 8145.1 ± 103.4 b | 1540.8 ± 21.8 c | 670.6 ± 44.1 a | 523.5 ± 1.8 a,b | 135.4 ± 2.5 c | 5.0 ± 0.4 b | 3.6 ± 0.3 a | 11.0 ± 0.2 | |
| 10 | HPLC 1 | 9570.5 ± 221.0 a | 2748.1 ± 68.6 a | 798.0 ± 16.0 b | 1252.1 ± 62.8 a | 140.6 ± 14.9 b | 3.9 ± 0.3 c | 1.2 ± 0.2 c | 14.5 ± 0.4 |
| HPLC 2 | 10473.9 ± 454.4 a | 1655.4 ± 82.2 b | 956.3 ± 16.7 a | 734.6 ± 2.3 c | 881.0 ± 12.2 a | 7.7 ± 0.0 a | 2.6 ± 0.0 b | 14.7 ± 0.6 | |
| EM | 9460.9 ± 169.8 a | 1499.7 ± 21.8 b | 911.9 ± 35.5 a | 694.6 ± 10.8 b | 80.7 ± 0.0 c | 5.6 ± 0.5 b | 3.2 ± 0.9 a | 12.7 ± 0.2 |
| Methods | OA | Concentration (mg/L) | Equation | R2 | Precision% | R% | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|---|---|---|
| HPLC 1 | Citric | 606.6–12411.00 | Y = 1879.80x + 8077.20 | 1.0000 | 0.76 | 100.4 | 0.61 | 2.02 |
| Pyruvic | 0.00–8.90 | Y = 31419.00x + 1474.70 | 0.9995 | 4.21 | 92.2 | 0.03 | 0.11 | |
| l-malic | 72.11–1730.69 | Y = 1731.50x + 1210.30 | 0.9999 | 1.72 | 101.2 | 0.64 | 1.60 | |
| Succinic | 128.88–1611.00 | Y = 945.26x − 1668.30 | 0.9999 | 0.76 | 99.4 | 1.75 | 5.84 | |
| Lactic | 1.68–84.00 | Y = 815.37x − 629.03 | 1.0000 | 3.66 | 91.1 | 1.93 | 6.45 | |
| Fumaric | 0.09–4.7 | Y = 199715x − 5007.3 | 0.9999 | 2.95 | 94.4 | 0.01 | 0.03 | |
| Acetic | 1.68–427.20 | Y = 802.29x − 355.28 | 1.0000 | 0.88 | 83.6 | 2.11 | 7.05 | |
| Citric | 426.24–10656.00 | Y = 1011.90x − 15648.00 | 1.0000 | 0.57 | 100.9 | 1.16 | 3.86 | |
| HPLC 2 | Pyruvic | 0.00–30.20 | Y = 13348.00x − 732.84 | 0.9999 | 1.28 | 100.3 | 0.08 | 0.28 |
| l-malic | 56.10–1402.4 | Y = 797.75x + 2302.9 | 0.9999 | 0.48 | 102.8 | 1.00 | 3.33 | |
| Succinic | 122.48–3062.00 | Y = 605.52x − 6762.2 | 0.9999 | 1.73 | 100.1 | 2.13 | 7.10 | |
| Lactic | 2.45–522.0 | Y = 475.82x + 1562.20 | 0.9998 | 0.26 | 100.7 | 1.04 | 3.46 | |
| Fumaric | 0.00–7.36 | Y = 134887.00x + 4235.6 | 0.9999 | 0.53 | 98.2 | 0.01 | 0.03 | |
| Acetic | 10.89–544.40 | Y = 540.39x + 476.03 | 0.9999 | 0.54 | 108.5 | 1.98 | 6.6 |
| Method | Organic Acid | Retention Time (min) | Precision % | LOD | LOQ | Recovery in Standards (%) | Recovery in Samples (%) | Fortified Recovery (%) |
|---|---|---|---|---|---|---|---|---|
| HPLC 1 | Citric | 7.5 | 2.22 | 0.65 | 2.18 | 93.2 ± 1.8 | 101.9 ± 3.7 | 103.6 ± 1.9 |
| Pyruvic | 8.5 | 5.01 | 0.03 | 0.11 | 97.7 ± 1.0 | 139.9 ± 34.9 | 120.7 ± 8.8 | |
| l-malic | 9.2 | 3.68 | 0.69 | 2.29 | 93.2 ± 1.8 | 107.0 ± 8.4 | 115.6 ± 2.0 | |
| Succinic | 11.2 | 2.59 | 1.73 | 6.42 | 90.8 ± 6.7 | 93.9 ± 4.7 | 94.7 ± 1.3 | |
| Lactic | 12.1 | 4.32 | 1.90 | 6.33 | 101.6 ± 4.2 | 71.4 ± 16.3 | 70.4 ± 3.5 | |
| Fumaric | 13.5 | 4.12 | 0.01 | 0.04 | 94.0 ± 2.6 | 92.5 ± 3.4 | 94.49 ± 0.3 | |
| Acetic | 14.5 | 4.48 | 2.14 | 7.13 | 98.6 ± 2.6 | 88.7 ± 16.6 | 81.8 ± 5.6 | |
| HPLC 2 | Citric | 9.9 | 2.36 | 1.17 | 3.90 | 99.2 ± 2.3 | 99.0 ± 1.4 | 100.9 ± 2.1 |
| Pyruvic | 6.1 | 3.23 | 0.08 | 0.28 | 94.0 ± 9.2 | 99.7 ± 4.0 | 100.3 ± 0.7 | |
| l-malic | 6.4 | 0.61 | 1.00 | 3.33 | 100.8 ± 0.1 | 100.2 ± 2.9 | 102.8 ± 0.1 | |
| Succinic | 10.9 | 5.62 | 2.21 | 7.38 | 96.2 ± 5.4 | 95.1 ± 1.8 | 100.1 ± 2.7 | |
| Lactic | 7.4 | 0.70 | 1.03 | 3.42 | 101.3 ± 3.2 | 100.6 ± 3.1 | 100.7 ± 0.8 | |
| Fumaric | 12.4 | 3.28 | 0.01 | 0.03 | 91.2 ± 8.1 | 94.7 ± 2.8 | 98.2 ± 7.3 | |
| Acetic | 7.8 | 2.06 | 1.92 | 6.40 | 103.1 ± 4.4 | 101.7 ± 9.4 | 108.5 ± 10.1 |
| Organic Acid | Citric | Succinic | l-malic | Acetic | Lactic | Pyruvic | Fumaric | TOA |
|---|---|---|---|---|---|---|---|---|
| Content (mg/g) | 26.95 ± 0.41 | 0.63 ± 0.05 | 3.29 ± 0.08 | 0.75 ± 0.13 | 0.06 ± 0.00 | 0.02 ± 0.00 | nd | 31.70 ± 0.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Du, J.; Li, J.; Li, M. Quantification of the Organic Acids in Hawthorn Wine: A Comparison of Two HPLC Methods. Molecules 2019, 24, 2150. https://doi.org/10.3390/molecules24112150
Han Y, Du J, Li J, Li M. Quantification of the Organic Acids in Hawthorn Wine: A Comparison of Two HPLC Methods. Molecules. 2019; 24(11):2150. https://doi.org/10.3390/molecules24112150
Chicago/Turabian StyleHan, Yingying, Jinhua Du, Jie Li, and Miaomiao Li. 2019. "Quantification of the Organic Acids in Hawthorn Wine: A Comparison of Two HPLC Methods" Molecules 24, no. 11: 2150. https://doi.org/10.3390/molecules24112150
APA StyleHan, Y., Du, J., Li, J., & Li, M. (2019). Quantification of the Organic Acids in Hawthorn Wine: A Comparison of Two HPLC Methods. Molecules, 24(11), 2150. https://doi.org/10.3390/molecules24112150