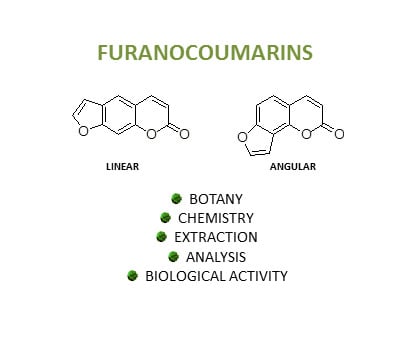
Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest
Abstract
:1. Biosynthesis of Furanocoumarins
1.1. Chemical Diversity in Natural Furanocoumarins
1.2. Localization in Tissues and Organs and Consequences for Sampling
1.3. Factors Affecting Furanocoumarin Content in Plants
2. Extraction of Furanocoumarins from Plants
3. Analysis of Furanocoumarins in Plants
4. Synthesis of Furanocoumarins
5. Biological Activities of Furanocoumarins
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stanjek, V.; Piel, J.; Boland, W. Synthesis of furanocoumarins: Mevalonate-independent prenylation of umbelliferone in Apium graveolens (Apiaceae). Phytochemistry 1999, 50, 1141–1145. [Google Scholar] [CrossRef]
- Hamerski, D.; Matern, U. Elicitor-induced biosynthesis of psoralens in Ammi Majus L suspension-cultures: Microsomal conversion of demethylsuberosin into (+)marmesin and psoralen. Eur. J. Biochem. 1988, 171, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.H.; Mendez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry; Johns Wiley & Sons: Chichester, UK, 1982. [Google Scholar]
- Santana, L.; Uriarte, E.F.; Roleira, F.; Milhazes, N.; Borges, F. Furocoumarins in medicinal chemistry. Synthesis, natural occurrence and biological activity. Curr. Med. Chem. 2004, 11, 3239–3261. [Google Scholar] [PubMed]
- Kitamura, N.; Kohtani, S.; Nakagaki, R. Molecular aspects of furocoumarin reactions: Photophysics, photochemistry, photobiology, and structural analysis. J. Photochem. Photobiol. C 2005, 6, 168–185. [Google Scholar] [CrossRef]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Innocenti, G.; Dall’Acqua, F.; Caporale, G. The role of 5,8-dihydroxypsoralen in the biosynthesis of isopimpinellin. Phytochemistry 1983, 22, 2207–2209. [Google Scholar] [CrossRef]
- Scott, B.R.; Pathak, M.A.; Mohn, G.R. Molecular and genetic basis of furocoumarin reactions. Mutat. Res. Genet. Toxicol. 1976, 39, 29–74. [Google Scholar] [CrossRef]
- Peroutka, R.; Schulzová, V.; Botek, P.; Hajšlová, J. Analysis of furanocoumarins in vegetables (Apiaceae) and citrus fruits (Rutaceae). J. Sci. Food Agric. 2007, 87, 2152–2163. [Google Scholar] [CrossRef]
- De Castro, W.V.; Mertens-Talcott, S.; Rubner, A.; Butterweck, V.; Derendorf, H. Variation of flavonoids and furanocoumarins in grapefruit juices: A potential source of variability in grapefruit juice− drug interaction studies. J. Agric. Food Chem. 2006, 54, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Schulzová, V.; Hajšlová, J.; Botek, P.; Peroutka, R. Furanocoumarins in vegetables: Influence of farming system and other factors on levels of toxicants. J. Sci. Food Agric. 2007, 87, 2763–2767. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Touillaud, M. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, J.; Zimmerli, B.; Dick, R.; Panizzon, R.; Schlatter, C.H. Dietary intake and risk assessment of phototoxic furocoumarins in humans. Food Chem. Toxicol. 1991, 29, 523–530. [Google Scholar] [CrossRef]
- Meleoug, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biolochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Olry, A.; Hehn, A. Recent advances in molecular genetics of furanocoumarin synthesis in higher plants. In Recent Advances in Redox Active Plant and Microbial Products; Jacob, C., Kirsch, G., Slusarenko, A.J., Winyard, P.G., Burkholz, T., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 363–375. [Google Scholar]
- Munakata, R.; Olry, A.; Karamat, F.; Courdavault, V.; Sugiyama, A.; Date, Y.; Grosjean, J. Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 2016, 211, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef]
- Fujita, K.I. Food-drug interactions via human cytochrome P450 3A (CYP3A). Drug Metab. Drug Interact. 2004, 20, 195–218. [Google Scholar] [CrossRef]
- Fracarolli, L.; Rodrigues, G.B.; Pereira, A.C.; Massola Júnior, N.S.; Silva-Junior, G.J.; Bachmann, L.; Wainwright, M.; Bastos, J.K.; Braga, G.U.L. Inactivation of plant-pathogenic fungus Colletotrichum acutatum with natural plant-produced photosensitizers under solar radiation. J. Photochem. Photobiol. B Biol. 2016, 162, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-Spiroudi, U.; Ntalli, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2015, 71, 1099–1105. [Google Scholar] [CrossRef]
- Meiners, T. Chemical ecology and evolution of plant–insect interactions: A multitrophic perspective. Curr. Opin. Insect Sci. 2015, 8, 22–28. [Google Scholar] [CrossRef]
- Zobel, A.M.; Brown, S.A.; Glowniak, K. Localization of furanocoumarins in leaves, fruits, and seeds of plants causing contact photodermatitis. Planta Med. 1990, 56, 571–572. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Chwil, M. Localisation of furanocoumarins in the tissues and on the surface of shoots of Heracleum sosnowskyi. Botany 2017, 95, 1057–1070. [Google Scholar] [CrossRef]
- Zobel, A.M.; Brown, S.A. Determination of furanocoumarins on the leaf surface of Ruta graveolens with an improved extraction technique. J. Nat. Prod. 1988, 51, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Zobel, A.M.; Brown, S.A. Furanocoumarins on the surface of callus cultures from species of the Rutaceae and Umbelliferae. Can. J. Bot. 1993, 71, 966–969. [Google Scholar] [CrossRef]
- Diwan, R.; Malpathak, N. Histochemical localization in Ruta graveolens cell cultures: Elucidating the relationship between cellular differentiation and furanocoumarin production. In Vitro Cell. Dev. Biol. Plant 2010, 46, 108–116. [Google Scholar] [CrossRef]
- Camm, E.L.; Wat, C.K.; Towers, G.H.N. An assessment of the roles of furanocoumarins in Heracleum lanatum. Can. J. Bot. 1976, 54, 2562–2566. [Google Scholar] [CrossRef]
- Zobel, A.M.; Brown, S.A. Furanocoumarin concentrations in fruits and seeds of Angelica archangelica. Environ. Exp. Bot. 1991, 31, 447–452. [Google Scholar] [CrossRef]
- Walker, D.J.; Martínez-Fernández, D.; Correal, E.; Romero-Espinar, P.; del Río, J.A. Accumulation of furanocoumarins by Bituminaria bituminosa in relation to plant development and environmental stress. Plant Physiol. Biochem. 2012, 54, 133–139. [Google Scholar] [CrossRef]
- Wszelaki, N.; Paradowska, K.; Jamróz, M.K.; Granica, S.; Kiss, A.K. Bioactivity-guided fractionation for the butyrylcholinesterase inhibitory activity of furanocoumarins from Angelica archangelica L. roots and fruits. J. Agric. Food Chem. 2011, 59, 9186–9193. [Google Scholar] [CrossRef]
- Zobel, A.M.; March, R.E. Autofluorescence reveals different histological localizations of furanocoumarins in fruit of some Umbelliferae and Leguminosae. Ann. Bot. 1993, 71, 251–255. [Google Scholar] [CrossRef]
- Zobel, A.M.; Brown, S.A. Histological localization of furanocoumarins in Ruta graveolens shoots. Can. J. Bot. 1989, 67, 915–921. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Caristi, C.; Leuzzi, U. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellocco, E.; Barreca, D.; Gattuso, G.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with therapeutic and health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar]
- Dercks, W.; Trumble, J.; Winter, C. Impact of atmospheric pollution on linear furanocoumarin content in celery. J. Chem. Ecol. 1990, 16, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Mamoucha, S.; Fokialakis, N.; Christodoulakis, N.S. Leaf structure and histochemistry of Ficus carica (Moraceae), the fig tree. Flora 2016, 218, 24–34. [Google Scholar] [CrossRef]
- Uckoo, R.M.; Jayaprakasha, G.K.; Balasubramaniam, V.M.; Patil, B.S. Grapefruit (Citrus paradisi Macfad) phytochemicals composition is modulated by household processing techniques. J. Food Sci. 2012, 77, C921–C926. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Buslig, B.S. Distribution of furanocoumarins in grapefruit juice fractions. J. Agric. Food Sci. 2005, 53, 5158–5163. [Google Scholar] [CrossRef] [PubMed]
- Zobel, A.M.; Brown, S.A. Seasonal changes of furanocoumarin concentrations in leaves of Heracleum lanatum. J. Chem. Ecol. 1990, 16, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Trumble, J.T.; Millar, J.G.; Ott, D.E.; Carson, W.C. Seasonal patterns and pesticidal effects on the phototoxic linear furanocoumarins in celery, Apium graveolens L. J. Agric. Food Chem. 1992, 40, 1501–1506. [Google Scholar] [CrossRef]
- Liang, W.H.; Chang, T.W.; Chang, Y.C. Influence of harvest stage on the pharmacological effect of Angelica dahurica. Bot. Stud. 2018, 59, 14. [Google Scholar] [CrossRef]
- Cancalon, P.F.; Barros, S.M.; Haun, C.; Widmer, W.W. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J. Food Sci. 2011, 76, C543–C548. [Google Scholar] [CrossRef]
- Reitz, S.R.; Karowe, D.N.; Diawara, M.M.; Trumble, J.T. Effects of elevated atmospheric carbon dioxide on the growth and linear furanocoumarin content of celery. J. Agric. Food Chem. 1997, 45, 3642–3646. [Google Scholar] [CrossRef]
- Nigg, H.N.; Strandberg, J.O.; Beier, R.C.; Petersen, H.D.; Harrison, J.M. Furanocoumarins in Florida celery varieties increased by fungicide treatment. J. Agric. Food Chem. 1997, 45, 1430–1436. [Google Scholar] [CrossRef]
- Dugrand-Judek, A.; Olry, A.; Hehn, A.; Costantino, G.; Ollitrault, P.; Froelicher, Y.; Bourgaud, F. The distribution of coumarins and furanocoumarins in citrus species closely matches citrus phylogeny and reflects the organization of biosynthetic pathways. PLoS ONE 2015, 10, e0142757. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Poutaraud, A.; Bourgaud, F.; Girardin, P.; Gontier, E. Cultivation of rue (Ruta graveolens L.; Rutaceae) for the production of furanocoumarins of therapeutic value. Can. J. Bot. 2000, 78, 1326–1335. [Google Scholar]
- Waksmundzka-Hajnos, M.; Petruczynik, A.; Dragan, A.; Wianowska, D.; Dawidowicz, A.D. Effect of extraction method on the yield of furanocoumarins from fruits of Archangelica officinalis Hoffm. Phytochem. Anal. 2004, 15, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Singh, D.P.; Singh, A.P.; Pandey, M.M.; Singh Rawat, A.K. A validated HPLC method for quantification and optimization of furocoumarins in different extracts of fruits of Heracleum candicans. Chromatogr. 2007, 66, 401–405. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Sun, J.; Han, J.; Guo, D.A. Chromatographic fingerprint analysis and characterization of furocoumarins in the roots of Angelica dahurica by HPLC/DAD/ESI-MSn technique. J. Pharm. Biomed. Anal. 2008, 47, 778–785. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, J.; Zhang, L.; Gao, B.; Shi, S.; Wang, X.; Li, J.; Tu, P. Simultaneous characterisation of fifty coumarins from the roots of Angelica dahurica by off-line two-dimensional high-performance liquid chromatography coupled with electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2014, 25, 229–240. [Google Scholar] [CrossRef]
- Chen, L.; Jian, Y.; Wei, N.; Mei, Y.; Zhuang, X.; Li, H. Separation and simultaneous quantification of nine furanocoumarins from Radix Angelicae dahuricae using liquid chromatography with tandem mass spectrometry for bioavailability determination in rats. J. Sep. Sci. 2015, 38, 4216–4224. [Google Scholar] [CrossRef]
- Shinde, P.B.; Ladda, K.S. Simultaneous quantification of furanocoumarins from Aegle marmelos fruit pulp extract. J. Chroamtogr. Sci. 2015, 53, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Picolella, S.; Galasso, S.; Fiorentino, A.; Kretschmer, N.; Pan, S.-P.; Bauer, R.; Monaco, P. Influence of harvest season on chemical composition and bioactivity of wild rue plant hydroalcoholic extracts. Food Chem. Toxicol. 2016, 90, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, I.; Murauer, A.; Ganzera, M. Determination of coumarins in roots of Angelica dahurica by supercritical fluid chromatography. J. Pharm. Biomed. Anal. 2016, 129, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, Y.; Tao, H.; Liao, L.; Li, Y.; Zhang, Z. Direct analysis in real-time mass spectrometry for rapid identification of traditional Chinese medicines with coumarins as primary characteristics. Phytochem. Anal. 2017, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Okiura, A.; Kohno, M. Phenylpropanoid composition in fig (Ficus carica L.) leaves. J. Nat. Med. 2017, 71, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Tine, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. A method for LC-MS/MS profiling of coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler extracts and essential oils. Molecules 2017, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Ušjak, L.J.; Drobac, M.M.; Niketić, M.S.; Petrović, S.D. Chemosystematic significance of essential oil constituents and furanocoumarins of underground parts and fruits of nine Heracleum L. taxa from southeastern Europe. Chem. Biodivers. 2018, 15, e1800412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-D.; Xu, X.; Lin, Y.-F.; Xia, H.-Y.; Huang, L.; Dong, M.-S. On-line screening and identification of free radical scavenging compounds in Angelica dahurica fermented with Eurotium cristatum using an HPLC-PDATriple-TOF-MS/MS-ABTS system. Food Chem. 2019, 272, 670–678. [Google Scholar] [CrossRef]
- Innocenti, G.; Piovan, A.; Filippini, R.; Caniato, R.; Cappelletti, E.M. Quantitative recovery of furanocoumarins from Psoralea bituminosa. Pytochem. Anal. 1997, 8, 84–86. [Google Scholar] [CrossRef]
- Järvenpää, E.P.; Jestoi, M.N.; Huopalahti, R. Quantitative determination of phototoxic furocoumarins in celeriac (Apium graveolens L. var. rapeceum) using supercritical fluid extraction and high performance liquid chromatography. Pytochem. Anal. 1997, 8, 250–256. [Google Scholar] [CrossRef]
- Nguyen, C.; Bouque, V.; Bourgaud, F.; Guckert, A. Quantification of daidzein and furanocoumarin conjugates of Psoralea cinerea L. (Leguminosae). Pytochem. Anal. 1997, 8, 27–31. [Google Scholar] [CrossRef]
- Scordino, M.; Sabatino, L.; Belligno, A.; Gagliano, G. Flavonoids and furocoumarins distribution of unripe chinotto (Citrus × myrtifolia Rafinesque) fruit: Beverage processing homogenate and juice characterization. Eur. Food Res. Technol. 2011, 233, 759–767. [Google Scholar] [CrossRef]
- Qiu, H.; Xiao, X.; Li, G. Separation and purification of furanocoumarins from Toddalia asiatica (L.) Lam. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J. Sep. Sci. 2012, 35, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Du, G.; He, H.; Xu, H.; Guo, D.; Li, R. Two nematicidal furocoumarins from Ficus carica L. leaves and their physiological effects on pine wood nematode (Bursaphelenchus xylophilus). Nat. Prod. Res. 2016, 30, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Chebrolu, K.K.; Jifon, J.; Patil, B.S. Modulation of flavanone and furocoumarin levels in grapefruits (Citrus paradisi Macfad.) by production and storage conditions. Food Chem. 2016, 196, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.; Liberto, E.; Beolor, J.-C.; Brevard, H.; Bicchi, C.; Rubiolo, P. Oxygenated heterocyclic compounds to differentiate Citrus spp. Essential oils through metabolomic strategies. Food Chem. 2016, 206, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-L.; Tang, S.-Y.; Liu, C.-B.; Ye, L.; Yang, P.-S.; Zhang, F.-M.; Liu, Z.-H.; Miao, M.-M.; Guo, Y.-D.; Shen, Q.-P. Three new benzolactones from Lavandula angustifolia and their bioactivities. J. Asian Nat. Prod. Res. 2016, 19, 766–773. [Google Scholar] [CrossRef]
- Xiong, Y.; Chang, M.; Deng, K.; Luo, Y. A new phenolic glycoside and two new monoterpenoid furocoumarins from Aurantii Fructus Immaturus. Nat. Prod. Res. 2016, 30, 1571–1576. [Google Scholar] [CrossRef]
- Yang, W.-Q.; Zhu, Z.-X.; Song, Y.-L.; Qi, B.-W.; Wang, J.; Su, C.; Tu, P.-F.; Shi, S.-P. Dimeric furanocoumarins from the roots of Angelica dahurica. Nat. Prod. Res. 2017, 31, 870–877. [Google Scholar] [CrossRef]
- Lin, C.-H.; Funayama, S.; Peng, S.-F.; Kuo, C.-L.; Chung, J.-G. The ethanol extraction of prepared Psoralea corylifolia induces apoptosis and autophagy and alteres genes expression assayed by cDNA microarray in human prostate cancer PC-3 cells. Environ. Toxicol. 2018, 33, 770–788. [Google Scholar] [CrossRef]
- Sarshar, S.; Sendker, J.; Qin, X.; Goycoolea, F.M.; Asadi Karam, M.R.; Habibi, M.; Bouzari, S.; Dobrindt, U.; Hensel, A. Antiadhesive hydroalcoholic extract from Apium graveolens fruits prevents bladder and kidney infection against uropathogenic E. coli. Fitoterapia 2018, 127, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ito, Y. Isolation of Imperatorin, oxypeucedanin, and isoimperatorin from Angelica dahurica (Fisch. Ex Hoffm) Benth. et Hook by stepwise flow rate high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1609–1618. [Google Scholar] [CrossRef]
- Skalicka-Woz’niak, K.; Mendel, M.; Chłopecka, M.; Dziekan, N. Isolation and evaluation of the myorelaxant effect of bergapten on isolated rat jejunum. Pharm. Biol. 2016, 54, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Suthiwong, J.; Thongsri, Y.; Yenjai, C. A new furanocoumarin from the fruits of Scaevola taccada and antifungal activity against Pythium insidiosum. Nat. Prod. Res. 2017, 31, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hasitha, P.; Dharmesh, S.M. Antioxidant and anti-inflammatory properties of marmelosin from Bael (Aegle marmelos L.); Inhibition of TNF-α mediated inflammatory/tumor markers. Biomed. Pharmacother. 2018, 106, 98–108. [Google Scholar]
- Saleh-e-In, M.; Roy, A.; Al-Mansur, M.A.; Hasan, C.M.; Rahim, M.; Sultan, N.; Ahmed, S.; Islam, R.; Van Staden, J. Isolation and in silico prediction of potential drug-like compounds from Anethum sowa L. root extracts targeted towards cancer therapy. Comput. Biol. Chem. 2019, 78, 242–259. [Google Scholar] [CrossRef]
- Kviesis, J.; Kļimenkovs, I.; Arbidans, L.; Podjava, A.; Kļaviņš, M.; Liepiņš, E. Evaluation of furanocoumarins from seeds of the wild parsnip (Pastinaca sativa L. s.l.). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1105, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiao, J.; Gai, Q.-Y.; Wang, P.; Guo, N.; Niu, L.-L.; Fu, Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Distribution of flavonoids and furocoumarins in juices from cultivars of Citrus bergamia Risso. J. Agric. Food Chem. 2007, 55, 9921–9927. [Google Scholar] [CrossRef]
- Vander Molen, K.M.; Cech, N.B.; Paine, M.F.; Oberlies, N.H. Rapid quantitation of furanocoumarins and flavonoids in grapefruit juice using Ultra Performance Liquid Chromatography. Phytochem. Anal. 2013, 24, 1–15. [Google Scholar]
- Santana, L.L.B.; Silva, C.V.; Almeida, L.C.; Costa, T.A.C.; Velozo, E.S. Extraction with supercritical fl uid and comparison of chemical composition from adults and young leaves of Zanthoxylum tingoassuiba. Brazillian J. Pharmacogn. 2011, 21, 564–567. [Google Scholar] [CrossRef]
- Zgórka, G.; Gtowniak, K. Simultaneous Determination of phenolic acids and linear furanocoumarins in Fruits of Libanotis dolichostyla by solid-phase extraction and High-Performance Liquid Chromatography. Phytochem. Anal. 1999, 10, 268–271. [Google Scholar] [CrossRef]
- Ostertag, E.; Becker, T.; Ammon, J.; Bauer-Aymanns, H.; Schrenk, D. Effects of storage conditions on furocoumarin levels in intact, chopped, or homogenized parsnips. J. Agric. Food Chem. 2002, 50, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Kočar, D. Different sample preparation methods combined with LC–MS/MS and LC–UV for determination of some furocoumarin compounds in products containing citruses. Flavour Fragr. J. 2008, 23, 263–271. [Google Scholar] [CrossRef]
- Li, G.-J.; Wu, H.-J.; Wang, Y.; Hung, W.-L.; Rousseff, R.L. Determination of citrus juice coumarins, furanocoumarins and methoxylated flavones using solid phase extraction and HPLC with photodiode array and fluorescence detection. Food Chem. 2019, 271, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Messer, A.; Nieborowski, A.; Strasser, C.; Lohr, C.; Schrenk, D. Major furocoumarins in grapefruit juice I: Levels and urinary metabolite(s). Food Chem. Toxicol. 2011, 49, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, K.; Vance, T.M.; Perkins, C.; Provatas, A.; Wu, S.; Qureshi, A.; Cho, E.; Chun, O.K. Development of a comprehensive analytical method for furanocoumarins in grapefruit and their metabolites in plasma and urine using UPLC-MS/MS: A preliminary study. Int. J. Food. Sci. Nutr. 2016, 67, 881–887. [Google Scholar] [CrossRef]
- Cook, D.W.; Burnham, M.L.; Harmes, D.C.; Stoll, D.R.; Rutan, S.C. Comparison of multivariate curve resolution strategies in quantitative LCxLC: Application to the quantification of furanocoumarins in apiaceous vegetables. Anal. Chim. Acta 2017, 961, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, O.M.; Campos Curcino Vieira, L.; Sayuri Bellete, B.; King-Diaz, B.; Lotina-Hennsen, B.; das Graças Fernandes da Silva, M.F.; Moura Veiga, T.A. Evaluation of alkaloids isolated from Ruta graveolens as photosynthesis inhibitors. Molecules 2018, 23, 2693. [Google Scholar] [CrossRef]
- Zaynoun, S.; Abi Ali, L.; Tenekjian, K.; Kurban, A. The bergapten content of garden parsley and its significance in causing cutaneous photosensitization. Clin. Exp. Dermatol. 1985, 10, 328–331. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Yang, X.-W. Simultaneous quantification of nine new furanocoumarins in Angelicae dahuricae radix using ultra-fast liquid chromatography with tandem mass spectrometry. Molecules 2017, 22, 322. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Yang, H.J.; Ma, J.Y. Simultaneous determination of three furanocoumarins by UPLC/MS/MS: Application to pharmacokinetic study of Angelica dahurica radix after oral administration to normal and experimental colitis-induced rats. Molecules 2017, 22, 416. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N.; Vogler, B.; Bates, R.B.; Schmidt, J.M.; Dicus, C.W.; Nakkiew, P.; Haber, W.A. HPLC-NMR/HPLC-MS analysis of the bark extract of Stauranthus perforatus. Phytochem. Anal. 2003, 14, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Corbi, E.; Pérès, C.; David, N. Quantification of furocoumarins in hydroalcoholic fragrances by a liquid chromatography–high resolution/accurate mass method. Flavour Fragr. J. 2014, 29, 173–183. [Google Scholar] [CrossRef]
- Jeong, M.; Hong, T.; Lee, K.; Hwangbo, H.; Kim, M.; Ma, W.; Zahn, M. HPLC method for simultaneous quantification of bakuchiol and minor furocoumarins in bakuchiol extract from Psoralea corylifolia. J. AOAC Int. 2015, 98, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Frérot, E.; Decorzant, E. Quantification of total furocoumarins in citrus oils by HPLC coupled with UV, fluorescence, and mass detection. J. Agric. Food Chem. 2004, 52, 6879–6886. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Mandrioli, R.; Ferranti, A.; Sorella, V.; Protti, M.; Epifano, F.; Curini, M.; Raggi, M.A. Quantitative evaluation of auraptene and umbelliferone, chemopreventive coumarins in citrus fruits, by HPLC-UV-FL-MS. J. Agric. Food Chem. 2013, 61, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Protti, M.; Valle, F.; Poli, F.; Raggi, M.A.; Mercolini, L. Bioactive molecules as authenticity markers of Italian Chinotto (Citrus × myrtifolia) fruits and beverages. J. Pharm. Biomed. Anal. 2015, 104, 75–80. [Google Scholar] [CrossRef]
- Desmortreux, C.; Rothaupt, M.; West, C.; Lesellier, E. Improved separation of furocoumarins of essential oils by supercritical fluid chromatography. J. Chromatogr. A 2009, 1216, 7088–7095. [Google Scholar] [CrossRef]
- Felix D’Mello, J.P. Handbook of Plant and Fungal Toxicants; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Sarker, S.D.; Nahar, L. Natural medicine: The genus Angelica. Curr. Med. Chem. 2004, 11, 1479–1500. [Google Scholar] [CrossRef]
- Melough, M.M.; Chun, O.K. Dietary furocoumarins and skin cancer: A review of current biological evidence. Food Chem. Toxicol. 2018, 122, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Malcolm, J.; Arnold, O.; Spence, J.D. Grapefruit juice–drug interactions. Br. J. Clin. Pharmacol. 2002, 46, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Dresser, G.K. Interactions between grapefruit juice and cardiovascular drugs. Am. J. Cardiovasc. Drugs 2004, 4, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G. Fruit juice inhibition of uptake transport: A new type of food-drug interaction. Br. J. Clin. Pharmacol. 2010, 70, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products. A Biosynthetic Approach, 3rd ed.; John Willey and Sons: Hoboken, NJ, USA, 2009; pp. 163–165.108. [Google Scholar]
- Mi, C.; Ma, J.; Wang, K.S.; Zuo, H.X.; Wang, Z.; Li, M.Y.; Piao, L.X.; Xu, G.H.; Li, X.; Quan, Z.S.; et al. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1 alpha via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol. 2017, 203, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.L.; Giordano, F. Effects of psoralens as anti-tumoral agents in breast cancer cells. World J. Clin. Oncol. 2014, 5, 348–358. [Google Scholar] [CrossRef]
- Wrześniok, D.; Beberok, A.; Rok, J.; Delijewski, M.; Hechmann, A.; Oprzondek, M.; Rzepka, Z.; Bacler-Zbikowska, B.; Buszman, E. UVA radiation augments cytotoxic activity of psoralens in melanoma cells. Int. J. Radiat. Biol. 2017, 93, 734–739. [Google Scholar] [CrossRef]
- Atilla, E.; Atilla, P.A.; Bozdag, S.C.; Yuksel, M.K.; Toprak, S.K.; Topcuoglu, P.; Akay, B.N.; Sanli, H.; Akan, H.; Demirer, T.; et al. Extracorporeal photochemotherapy in mycosis fungoides. Transfus. Clin. Biol. 2017, 24, 454–457. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.S.; Kim, S.-H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, E.J.; Lee, J.H.; Yang, W.M.; Nam, D.; Lee, J.H.; Lee, S.G.; Um, J.Y.; Shim, B.S.; Ahn, K.S. Simvastatin in combination with bergamottin potentiates TNF-induced apoptosis through modulation of NF-kappa B signalling pathway in human chronic myelogenous leukaemia. Pharm. Biol. 2016, 54, 2050–2060. [Google Scholar] [CrossRef]
- Cai, Y.; Kleiner, H.; Johnston, D.; Dubowski, A.; Bostic, S.; Ivie, W.; DiGiovanni, J. Effect of naturally occurring coumarins on the formation of epidermal DNA adducts and skin tumors induced by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis 1997, 18, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Washida, K.; Murakami, A. Suppressive effects of selected food phytochemicals on CD74 expression in NCI-N87 gastric carcinoma cells. J. Clin. Biochem. Nutr. 2008, 43, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Ferlazzo, N.; Cirmi, S.; Trapasso, E.; Bramanti, P.; Lombardo, G.E.; Minciullo, P.L.; Calapai, G.; Gangemi, S. Effects of bergamot essential oil and its extractive fractions on SH-SY5Y human neuroblastoma cell growth. J. Pharm. Pharmacol. 2015, 67, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.; Giordano, F.; Palma, M.; Bartella, V.; Rago, V.; Maggiolini, M.; Sisci, D.; Lanzino, M.; De Amicis, F.; Ando, S. Evidence that Bergapten, independently of its Photoactivation, Enhances p53 Gene Expression and Induces Apoptosis in Human Breast Cancer Cells. Curr. Cancer Drug Targets 2009, 9, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.L.; Giordano, F.; Rizza, P.; Pellegrino, M.; Zito, D.; Giordano, C.; Mauro, L.; Catalano, S.; Aquila, S.; Sisci, D. Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res. Treat. 2012, 136, 443–455. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Aquila, S.; Morelli, C.; Guido, C.; Santoro, M.; Perrotta, I.; Mauro, L.; Giordano, F.; Nigro, A.; Andò, S.; et al. Bergapten drives autophagy through the up-regulation of PTEN expression in breast cancer cells. Mol. Cancer 2015, 14, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.C.; Qu, X.; Yu, H.F.; Zhang, H.M.; Wang, Z.H.; Zhang, Z.T. Antitumor and apoptotic effects of bergaptol are mediated via mitochondrial death pathway and cell cycle arrest in human breast carcinoma cells. Bangladesh J. Pharmacol. 2016, 11, 489–494. [Google Scholar] [CrossRef]
- Razavi, S.M.; Zahri, S.; Motamed, Z.; Ghasemi, G. Bioactivity of Malva sylvestris L., a medicinal plant from Iran. Iranian J. Basic Med. Sci. 2010, 13, 133–138. [Google Scholar]
- Shalaby, N.M.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins. BioMed Res. Int. 2014, 5, 480545. [Google Scholar]
- Shin, K.H.; Chung, M.S.; Cho, T.S. Effects of furanocoumarins from Angelica dahurica on aldose reductase and galactosemic cataract formation in rats. Arch. Pharm. Res. 1994, 17, 331–336. [Google Scholar] [CrossRef]
- Hsu, F.-L.; Lai, C.-W.; Cheng, J.-T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Med. 1997, 63, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food. Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl)glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chem. 2011, 124, 576–582. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid profile and radical scavenging activity of Mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chem. 2011, 129, 1504–1512. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid composition and antioxidant activity of juices from chinotto (Citrus × myrtifolia Raf.) fruits at different ripening stages. J. Agric. Food Chem. 2010, 58, 3031–3036. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Tellone, E.; Ficarra, S.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Influences of flavonoids on erythrocyte membrane and metabolic implication through anionic exchange modulation. J. Membr. Biol. 2009, 230, 163–171. [Google Scholar] [CrossRef]
- Bellocco, E.; Barreca, D.; Laganà, G.; Leuzzi, U.; Tellone, E.; Kotyk, A.; Galtieri, A. Influence of L-rhamnosyl-D-glucosyl derivatives on properties and biological interaction of flavonoids. Mol. Cell. Biochem. 2009, 321, 165–171. [Google Scholar] [CrossRef]
- Piao, X.L.; Park, I.H.; Baek, S.H.; Kim, H.Y.; Park, M.K.; Park, J.H. Antioxidative activity of furanocoumarins isolated from Angelicae dahuricae. J. Ethnopharm. 2004, 93, 243–246. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Surapinit, S.; Tippyang, S. A novel furanocoumarin from Feroniella lucida exerts protective effect against lipid peroxidation. Phytother. Res. 2006, 20, 708–710. [Google Scholar] [CrossRef]
- Girennavar, B.; Jayaprakasha, G.; Jadegoud, Y.; Gowda, G.N.; Patil, B.S. Radical scavenging and cytochrome P450 3A4 inhibitory activity of bergaptol and geranylcoumarin from grapefruit. Bioorg. Med. Chem. 2007, 15, 3684–3691. [Google Scholar] [CrossRef]
- Bai, Y.; Li, D.; Zhou, T.; Qin, N.; Li, Z.; Yu, Z.; Hua, H. Coumarins from the roots of Angelica dahurica with antioxidant and antiproliferative activities. J. Funct. Foods 2016, 20, 453–462. [Google Scholar] [CrossRef]
- Qian, G.-S.; Wang, Q.; Leung, K.S.-Y.; Qin, Y.; Zhao, Z.; Jiang, Z.-H. Quality assessment of Rhizoma et Radix Notopterygii by HPTLC and HPLC fingerprinting and HPLC quantitative analysis. J. Pharm. Biomed. Anal. 2007, 44, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Ho, C.T.A. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Tung, N.H.; Taniyama, R.; Miyanowaki, T.; Morinaga, O.; Shoyama, Y. Anti-inflammatory Activity of constituents isolated from aerial part of Angelica acutiloba Kitagawa. Phytother. Res. 2015, 29, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lai, J.E.; Chen, L.G.; Yen, K.Y.; Yang, L.L. Inducible nitric oxide synthase inhibitors of Chinese herbs. Part 2: Naturally occurring furanocoumarins. Bioorg. Med. Chem. 2000, 8, 2701–2707. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ahn, E.M.; Song, M.C.; Kim, D.W.; Kang, J.H.; Kwon, O.S.; Kang, T.C.; Baek, N.I. In vitro GABA-transaminase inhibitory compounds from the root of Angelica dahurica. Phytother. Res. 2005, 19, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Głowniak, K.; Czuczwar, S.J. Imperatorin enhances the protective activity of conventional antiepileptic drugs against maximal electroshock-induced seizures in mice. Eur. J. Pharmacol. 2007, 574, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Głowniak, K.; Czuczwar, S.J. Time-course and dose-response relationships of imperatorin in the mouse maximal electroshock seizure threshold model. Neurosci. Res. 2007, 59, 18–22. [Google Scholar] [CrossRef]
- Brandt, C.; Bethmann, K.; Gastens, A.M.; Löscher, W. The multidrug transporter hypothesis of drug resistance in epilepsy: Proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol. Dis. 2006, 24, 202–211. [Google Scholar] [CrossRef]
- Meng, F.H.; Xiong, Z.L.; Sun, Y.; Li, F. Coumarins from Cnidium monnieri (L.) and their proliferation stimulating activity on osteoblast-like UMR106 cells. Pharmazie 2004, 59, 643–645. [Google Scholar]
- Tang, C.H.; Yang, R.S.; Chien, M.Y.; Chen, C.C.; Fu, W.-M. Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and ERK-dependent pathway in osteoblasts. Eur. J. Pharmacol. 2008, 579, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Xiao, G. Regulation of the osteoblast-specific transcription factor, Runx2: Responsiveness to multiple signal transduction pathways. J. Cell Biochem. 2003, 88, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.Q.; Sicheri, F.; Howard, A.J.; Yang, D.S. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 2003, 425, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.J.; Zhao, W.J.; Zhang, X.T.; Zhao, W.L.; Wang, X.X.; Yin, S.H.; Jiang, F.; Zhao, Y.X.; Chen, F.N.; Li, S.L. Bergapten promotes bone marrow stromal cell differentiation into osteoblasts in vitro and in vivo. Mol. Cell. Biochem. 2015, 409, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ge, Y.; Li, H.; Yan, M.; Zhou, J.; Zhang, Y. Bergapten prevents lipopolysaccharide mediated osteoclast formation, bone resorption and osteoclast survival. Int. Orthop. 2014, 38, 627–634. [Google Scholar] [CrossRef]
- Deyhim, F.; Garica, K.; Lopez, E.; Gonzalez, J.; Ino, S.; Garcia, M.; Patil, B.S. Citrus juice modulates bone strength in male senescent rat model of osteoporosis. Nutrition 2006, 22, 559–563. [Google Scholar] [CrossRef]
- Deyhim, F.; Mandadi, K.; Faraji, B.; Patil, B.S. Grapefruit juice modulates bone quality in rats. J. Med. Food 2008, 11, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Deyhim, F.; Mandadi, K.; Patil, B.S.; Faraji, B. Grapefruit pulp increases antioxidant status and improves bone quality in orchidectomized rats. Nutrition 2008, 24, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Vander Molen, K.M.; Ainslie, G.R.; Paine, M.F.; Oberlies, N.H. Labeled content of two furanocoumarins in dietary supplements correlates with neither actual content nor CYP3A inhibitory activity. J. Pharm. Biomed. Anal. 2014, 98, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Compound | Biological Activity | References |
|---|---|---|
 | Photosensitizer, anti-proliferative and anti-hyperglycemic | [5,89,109,111,123] |
| Psoralen | ||
 | Cytoprotective, anti-proliferative, apoptotic agent, antibacterial, modulator of oral drug bioavailability, photosensitizer | [104,105,106,107,112,113,114,115] |
| Bergamottin | ||
 | Antioxidant, anti-inflammatory, anti-proliferative, apoptotic agent | [112,120,133,134,137] |
| Bergaptol | ||
 | Photosensitizer, inducer of cell autophagy, antioxidant, anti-inflammatory, anti-proliferative, analgesic and anti-coagulant; bone health promotion enhancer | [89,112,117,118,119,120,125,132,136,137,138,139,143,144,145,146,147,148,149,150,151] |
| Bergapten | ||
 | Antioxidant, anti-inflammatory, anti-proliferative, antibacterial, antifungal, antiarrhythmic, allelopatic and anti-estrogenic agent | [121,123,131,136,137,138,139] |
| Oxypeucedanin | ||
 | Antioxidant and anti-hyperglycemic | [123,131] |
| Oxypeucedanin hydrate | ||
 | Anti-hyperglycemic | [123] |
| Isooxypeucedanin | ||
 | Antioxidant, GABA level enhancer, anticonvulsant drug action enhancer | [123,139,140,141,142] |
| Imperatorin | ||
 | Antioxidant, anti-inflammatory, anti-proliferative, analgesic and anticoagulant | [131,136,137,138,139] |
| Isoimperatorin | ||
 | Antioxidant and anti-hyperglycemic | [123,131] |
| Byakangelicin | ||
 | Antioxidant | [131] |
| Byakangelicol | ||
 | Antioxidant | [131] |
| Neobyakangelicol | ||
 | Anti-hyperglycemic | [124] |
| Pangelin | ||
 | Antioxidant | [131] |
| Phellotorin | ||
 | Anti-inflammatory, anti-proliferative, analgesic and anticoagulant | [136,137,138,139,140,141] |
| Notopterol |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules 2019, 24, 2163. https://doi.org/10.3390/molecules24112163
Bruni R, Barreca D, Protti M, Brighenti V, Righetti L, Anceschi L, Mercolini L, Benvenuti S, Gattuso G, Pellati F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules. 2019; 24(11):2163. https://doi.org/10.3390/molecules24112163
Chicago/Turabian StyleBruni, Renato, Davide Barreca, Michele Protti, Virginia Brighenti, Laura Righetti, Lisa Anceschi, Laura Mercolini, Stefania Benvenuti, Giuseppe Gattuso, and Federica Pellati. 2019. "Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest" Molecules 24, no. 11: 2163. https://doi.org/10.3390/molecules24112163
APA StyleBruni, R., Barreca, D., Protti, M., Brighenti, V., Righetti, L., Anceschi, L., Mercolini, L., Benvenuti, S., Gattuso, G., & Pellati, F. (2019). Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules, 24(11), 2163. https://doi.org/10.3390/molecules24112163