Metal-Free Air Oxidation in a Convenient Cascade Approach for the Access to Isoquinoline-1,3,4(2H)-triones
Abstract
:1. Introduction
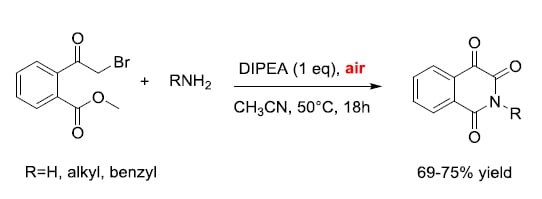
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Isoquinoline-1,3,4(2H)-triones Synthesis
3.3. Product Characterization
3.4. General Procedure for Isoquinoline-1,3,4(2H)-trione Synthesis via Staudinger Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chanda, T.; Zhao, J.C.-G. Recent Progress in Organocatalytic Asymmetric Domino Transformations. Adv. Synth. Catal. 2018, 360, 2–79. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Lai, B.; Gu, Y. Aldo-X Bifunctional Building Blocks for the Synthesis of Heterocycles. Chem. Rec. 2017, 17, 142–183. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Di Martino, M.; Capaccio, V.; Pierri, G.; Palombi, L.; Tedesco, C.; Massa, A. Synthesis of 2-Acetylbenzonitriles and Their Reactivity in Tandem Reactions with Carbon and Hetero Nucleophiles: Easy Access to 3,3-Disubstituted Isoindolinones. Eur. J. Org. Chem. 2018, 2018, 1699–1708. [Google Scholar] [CrossRef]
- Romano, F.; Di Mola, A.; Palombi, L.; Tiffner, M.; Waser, M.; Massa, A. Synthesis and Organocatalytic Asymmetric Nitro-aldol Initiated Cascade Reactions of 2-Acylbenzonitriles Leading to 3,3-Disubstituted Isoindolinones. Catalysts 2019, 9, 327. [Google Scholar] [CrossRef]
- Di Mola, A.; Macchia, A.; Tedesco, C.; Pierri, G.; Palombi, L.; Filosa, R.; Massa, A. Synthetic Strategies and Cascade Reactions of 2-Cyanobenzophenones for the Access to Diverse 3,3-Disubstituted Isoindolinones and 3-Aryl-3-Hydroxyisoindolinones. ChemistrySelect 2019, 4, 4820–4826. [Google Scholar] [CrossRef]
- Di Mola, A.; Palombi, L.; Massa, A. An overview on asymmetric synthesis of 3-substituted indolinones. Targets Heterocycl. Syst. 2014, 18, 113–140. [Google Scholar]
- Li, J.; Zhao, Y.-F.; Yuan, X.-Y.; Xu, J.-X.; Gong, P. Synthesis and Anticancer Activities of Novel 1,4-Disubstituted Phthalazines. Molecules 2006, 11, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Li, J.; He, L.; Zheng, S.; Bin Zhang, Y.; Gong, P. Synthesis and in vitro cytotoxicity of novel 1,4-disubstituted phthalazines. Chin. Chem. Lett. 2008, 19, 29–32. [Google Scholar] [CrossRef]
- Carnovale, I.M.; Lolli, M.L.; Serra, S.C.; Mingo, A.F.; Napolitano, R.; Boi, V.; Guidolin, N.; Lattuada, L.; Tedoldi, F.; Baranyai, Z.; et al. Exploring the intramolecular catalysis of the proton exchange process to modulate the relaxivity of Gd(iii)-complexes of HP-DO3A-like ligands. Chem. Commun. 2018, 54, 10056–10059. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Zhang, Y.-H.; Zhang, H.-J.; Liu, D.-Z.; Gu, M.; Li, J.-Y.; Wu, F.; Zhu, X.-Z.; Li, J.; Nan, F.-J. Design, Synthesis, and Biological Evaluation of Isoquinoline-1,3,4-trione Derivatives as Potent Caspase-3 Inhibitors. J. Med. Chem. 2006, 49, 1613–1623. [Google Scholar] [CrossRef]
- Ma, X.-Q.; Zhang, H.-J.; Zhang, Y.-H.; Chen, Y.-H.; Wu, F.; Du, J.-Q.; Yu, H.-P.; Zhou, Z.-L.; Li, J.-Y.; Nan, F.-J.; et al. Novel irreversible caspase-1 inhibitor attenuates the maturation of intracellular interleukin-1β. Biochem. Cell Boil. 2007, 85, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.-J.; Li, J.; Chen, L.-H.; Zhang, Y.-H.; Gu, M.; Zhang, H.-J. Isoquinoline-1,3,4-trione compounds, the method and the use thereof. US 20060135557, 2006. [Google Scholar]
- Zhang, Y.H.; Zhang, H.J.; Wu, F.; Chen, Y.H.; Ma, X.Q.; Du, J.Q.; Zhou, Z.L.; Li, J.Y.; Nan, F.J.; Li, J. Isoquinoline-1,3,4-trione and its derivatives attenuate beta-amyloid-induced apoptosis of neuronal cells. FEBS J. 2006, 273, 4842–4852. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Clarke, E.D.; Ridley, S.M.; Greenhow, D.T.; Gillen, K.J.; Vohra, S.K.; Wardman, P. 1,3,4(2H)-isoquinolinetrione herbicides: Novel redox mediators of photosystem I. Pestic. Sci. 1995, 44, 49–58. [Google Scholar] [CrossRef]
- Mitchell, G.; Clarke, E.D.; Ridley, S.M.; Bartlett, D.W.; Gillen, K.J.; Vohra, S.K.; Greenhow, D.T.; Ormrod, J.C.; Wardman, P. 1,3,4(2H)-Isoquinolinetriones: Evaluation of amino-substituted derivatives as redox mediator herbicides. Pest Manag. Sci. 2000, 56, 120–126. [Google Scholar] [CrossRef]
- Mitchell, G.; Clarke, E.D.; Ridley, S.M.; Gillen, K.J.; Vohra, S.K.; Greenhow, D.T. Synthesis and characterisation of some 4-keto derivatives of 1,3,4(2H)-isoquinolinetrione redox mediator herbicides. Pest Manag. Sci. 2000, 56, 127–132. [Google Scholar] [CrossRef]
- Mazza, M.; Modena, T. Herbicidal activity of 2-substituted 1,3,4-(2H)-isoquinolinetriones. Il Farm. 1999, 54, 339–345. [Google Scholar] [CrossRef]
- Quattropani, A.; Dorbais, J.; Covini, D.; Pittet, P.-A.; Colovray, V.; Thomas, R.J.; Coxhead, R.; Halazy, S.; Scheer, A.; Missotten, M.; et al. Discovery and Development of a New Class of Potent, Selective, Orally Active Oxytocin Receptor Antagonists. J. Med. Chem. 2005, 48, 7882–7905. [Google Scholar] [CrossRef] [PubMed]
- Pollers-Wieërs, C.; Vekemans, J.; Toppet, S.; Hoornaert, G. The use of isoquinolinetriones in the synthesis of benzo[c]phenanthridine alkaloids. Tetrahedron 1981, 37, 4321–4326. [Google Scholar] [CrossRef]
- Wakchaure, P.B.; Easwar, S.; Puranik, V.G.; Argade, N.P. Facile air-oxidation of N-homopiperonyl-5,6-dimethoxyhomophthalimide: Simple and efficient access to nuevamine. Tetrahedron 2008, 64, 1786–1791. [Google Scholar] [CrossRef]
- Yoshifuji, S.; Arakawa, Y. Ruthenium Tetraoxide Oxidation of 3,4-Dihydroisoquinolin-1(2H)-ones: An Efficient Synthesis of Isoquinoline-1,3,4(2H)-triones. Chem. Pharm. Bull. 1989, 37, 3380–3381. [Google Scholar] [CrossRef]
- Ling, K.-Q.; Ye, J.-H.; Chen, X.-Y.; Ma, D.-J.; Xu, J.-H. On the reactions of 1,3-isoquinolinediones with singlet oxygen. Tetrahedron 1999, 55, 9185–9204. [Google Scholar] [CrossRef]
- Ling, K.-Q.; Ji, G.; Cai, H.; Xu, J.-H. Dye-Sensitized Photooxygenations of 1,3-Isoquinolinediones. Tetrahedron Lett. 1999, 39, 2381–2384. [Google Scholar]
- Vekemans, J.; Hoornaert, G. A new pathway to 1,3,4(2H)-isoquinolinetriones and substituted isoindolinones. Tetrahedron 1980, 36, 943–950. [Google Scholar] [CrossRef]
- Mahajan, S.; Sharma, B.; Kapoor, K.K. A solvent-free one step conversion of ketones to amides via Beckmann rearrangement catalysed by FeCl3·6H2O in presence of hydroxylamine hydrochloride. Tetrahedron Lett. 2015, 56, 1915–1918. [Google Scholar] [CrossRef]
- Yadav, J.; Reddy, B.S.; Reddy, U.S.; Praneeth, K. Azido-Schmidt reaction for the formation of amides, imides and lactams from ketones in the presence of FeCl3. Tetrahedron Lett. 2008, 49, 4742–4745. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, W.-K.; Yang, L.; Ma, D.-Y. Iodine-catalyzed oxidative multiple C–H bond functionalization of isoquinolines with methylarenes: an efficient synthesis of isoquinoline-1,3,4(2H)-triones. Org. Biomol. Chem. 2017, 15, 7112–7116. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Alswaidan, I.; Marzouk, M.; Iba, M. Cytotoxicity Evaluation of a New Set of 2-Aminobenzo[de]iso-quinoline-1,3-diones. Int. J. Mol. Sci. 2014, 15, 22483–22491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsou, H.R.; Otteng, M.; Tran, T.; Floyd, M., Jr.; Reich, M.; Birnberg, G.; Kutterer, K.; Ayral-Kaloustian, S.; Ravi, M.; Nilakantan, R.; et al. 4-(Phenylaminomethylene)isoquinoline-1,3(2H,4H)-diones as potent and selective inhibitors of the cyclin-dependent kinase 4 (CDK4). J. Med. Chem. 2008, 51, 3507–3525. [Google Scholar] [CrossRef] [PubMed]
- Billamboz, M.; Bailly, F.; Lion, C.; Touati, N.; Vezin, H.; Calmels, C.; Andréola, M.-L.; Christ, F.; Debyser, Z.; Cotelle, P. Magnesium Chelating 2-Hydroxyisoquinoline-1,3(2H,4H)-diones, as Inhibitors of HIV-1 Integrase and/or the HIV-1 Reverse Transcriptase Ribonuclease H Domain: Discovery of a Novel Selective Inhibitor of the Ribonuclease H Function. J. Med. Chem. 2011, 54, 1812–1824. [Google Scholar] [CrossRef]
- Ontoria, J.M.; Rydberg, E.H.; Di Marco, S.; Tomei, L.; Attenni, B.; Malancona, S.; Hernando, J.I.M.; Gennari, N.; Koch, U.; Narjes, F.; et al. Identification and Biological Evaluation of a Series of 1H-Benzo[de]isoquinoline-1,3(2H)-diones as Hepatitis C Virus NS5B Polymerase Inhibitors‡. J. Med. Chem. 2009, 52, 5217–5227. [Google Scholar] [CrossRef]
- Vernekar, S.K.V.; Liu, Z.; Nagy, E.; Miller, L.; Kirby, K.A.; Wilson, D.J.; Kankanala, J.; Sarafianos, S.T.; Parniak, M.A.; Wang, Z. Design, Synthesis, Biochemical, and Antiviral Evaluations of C6 Benzyl and C6 Biarylmethyl Substituted 2-Hydroxylisoquinoline-1,3-diones: Dual Inhibition against HIV Reverse Transcriptase-Associated RNase H and Polymerase with Antiviral Activities. J. Med. Chem. 2015, 58, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Krawiecka, M.; Kossakowski, J.; Szymanek, K.; Kierzkowska, M.; Młynarczyk, G.; Kuran, B. Synthesis and antimicrobial activity of derivatives of 1H-benzo[de]isoquinoline-1,3(2H)-dione. Heterocycl. Commun. 2012, 18, 275–278. [Google Scholar]
- Staudinger, H.; Meyer, J. Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine. Helv. Chim. Acta 1919, 635–646. [Google Scholar] [CrossRef]
- Di Mola, A.; Scorzelli, F.; Monaco, G.; Palombi, L.; Massa, A. Highly diastereo- and enantioselective organocatalytic synthesis of new heterocyclic hybrids isoindolinone-imidate and isoindolinone-phthalide. RSC Adv. 2016, 6, 60780–60786. [Google Scholar] [CrossRef]
- Thapa, P.; Corral, E.; Sardar, S.; Pierce, B.S.; Foss, F.W., Jr. Isoindolinone Synthesis: Selective Dioxane-Mediated Aerobic Oxidation of Isoindolines. J. Org. Chem. 2019, 84, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Cheng, C.; Yang, G.; Wan, S.; Zhang, J.; Mao, Y.; Zhao, Y.; Zhang, L.; Li, C.; et al. Reductant-Free Aerobic Hydroxylation of Isoquinoline-1,3(2H,4H)-dione Derivatives. J. Org. Chem. 2019, 84, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Muchowski, J.M. One-step conversion of isatins to oxindoles and phthalonimides to homophthalimides. Can. J. Chem. 1969, 47, 857–859. [Google Scholar] [CrossRef]
- Zeneca, A. Isoquinolinetrione derivatives as herbicides. Pat. appln PCT/GB/94/01094, 1994. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |







| Entry | Base (1 eq) | T (°C) | Time (h) | Yield 3a a |
|---|---|---|---|---|
| 1 | - | r.t. | 3 | -- |
| 2 | - | r.t. | 18 | -- |
| 3 | DIPEA | r.t | 18 | 18% b |
| 4 | DIPEA | 50 | 3 | 53% |
| 5 | DIPEA | 50 | 18 | 71% |
| 6c | DIPEA | 50 | 18 | 10% |
| 7 | Et3N | 50 | 18 | 50% |
| 8 | NaHCO3 | r.t | 18 | 26% |
| 9 | NaHCO3 | 50 | 3 | 41% |
| 10 | K2CO3 | 50 | 4 | 40% |
| 11 | K2CO3 | 50 | 18 | Dec. |

| Entry | R | 3 | Yield % a |
|---|---|---|---|
| 1 | -CH2Ph | (3a) | 71% |
| 2 | 4-Cl-CH2Ph | (3b) | 73% |
| 3 | 4-MeO-CH2Ph | (3c) | 75% |
| 4 | 2-F-CH2Ph | (3d) | 69% |
| 5 | 3-NO2-CH2Ph | (3e) | 69% |
| 6 | -CH3 | (3f) | 72% |
| 7 | -CH2CH3 | (3g) | 70% |
| 8 9 | -(CH2)3CH3 -CH2CH=CH2 | (3h) (3i) | 73% 72% |
| 10 | Ph | -- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, A.; Tedesco, C.; Massa, A. Metal-Free Air Oxidation in a Convenient Cascade Approach for the Access to Isoquinoline-1,3,4(2H)-triones. Molecules 2019, 24, 2177. https://doi.org/10.3390/molecules24112177
Di Mola A, Tedesco C, Massa A. Metal-Free Air Oxidation in a Convenient Cascade Approach for the Access to Isoquinoline-1,3,4(2H)-triones. Molecules. 2019; 24(11):2177. https://doi.org/10.3390/molecules24112177
Chicago/Turabian StyleDi Mola, Antonia, Consiglia Tedesco, and Antonio Massa. 2019. "Metal-Free Air Oxidation in a Convenient Cascade Approach for the Access to Isoquinoline-1,3,4(2H)-triones" Molecules 24, no. 11: 2177. https://doi.org/10.3390/molecules24112177
APA StyleDi Mola, A., Tedesco, C., & Massa, A. (2019). Metal-Free Air Oxidation in a Convenient Cascade Approach for the Access to Isoquinoline-1,3,4(2H)-triones. Molecules, 24(11), 2177. https://doi.org/10.3390/molecules24112177