Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson’s Disease
Abstract
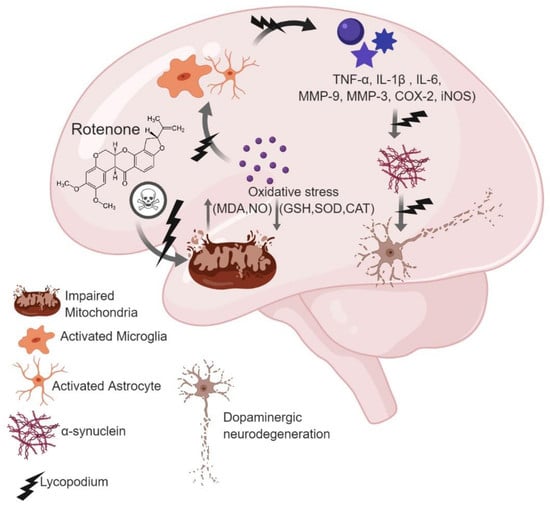
:1. Introduction
2. Results
2.1. Administration of Lyc Diminished Lipid Peroxidation and Enhanced Glutathione Levels in Rotenone Treated Rats
2.2. Lycopodium Reduced Rotenone-Induced Antioxidant Loss and Nitrite Content
2.3. Lyc Mitigates Pro-Inflammatory Cytokines Expression
2.4. Lyc Offers Neuroprotection by Inhibiting Matrix Metalloproteinase Expression
2.5. Lyc Exerts Neuroprotection by Modulating Glial Response
2.6. Effect of Lyc on Alpha-Synuclein Expression
2.7. Lyc Administration Diminished COX-2 and iNOS Expression
2.8. Lyc Protects Dopaminergic Neurons from Rotenone Induced Neurodegeneration
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Experimental Animals and Procedure
4.3. Tissue Preparation for Biochemical Studies
4.4. Biochemical Studies
4.5. Estimation of Lipid Peroxidation
4.6. Estimation of Reduced Glutathione
4.7. Estimation of the Activities of Antioxidant Enzymes
4.8. Estimation of Nitrite Levels
4.9. Estimation of Proinflammatory Cytokines and MMP-3 by ELISA Assays
4.10. Immunofluorescence Staining of GFAP and Iba-1
4.11. Assessment of Activated Astrocytes and Microglia in the Striatum
4.12. Western Blot Analysis of MMP-3, α-synuclein, COX-2 and iNOS
4.13. Immunohistochemistry for Tyrosine Hydroxylase (TH) Expression
4.14. Assessment of TH-ir Dopaminergic Neurons and TH-ir Dopamine Nerve Fibers Loss
4.15. Protein Estimation
4.16. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting alpha-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef]
- Franco, R.; Li, S.; Rodriguez-Rocha, H.; Burns, M.; Panayiotidis, M.I. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2010, 188, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Badger, J.L.; Cordero-Llana, O.; Hartfield, E.M.; Wade-Martins, R. Parkinson’s disease in a dish—Using stem cells as a molecular tool. Neuropharmacology 2014, 76, 88–96. [Google Scholar] [CrossRef]
- Gibson, G.E.; Huang, H.M. Mitochondrial enzymes and endoplasmic reticulum calcium stores as targets of oxidative stress in neurodegenerative diseases. J. Bioenerg. Biomembr. 2004, 36, 335–340. [Google Scholar] [CrossRef]
- Yacoubian, T.A.; Standaert, D.G. Targets for neuroprotection in Parkinson’s disease. Biochim. Biophys. Acta 2009, 1792, 676–687. [Google Scholar] [CrossRef]
- Ferrer, I. Early involvement of the cerebral cortex in Parkinson’s disease: Convergence of multiple metabolic defects. Prog. Neurobiol. 2009, 88, 89–103. [Google Scholar] [CrossRef]
- Ma, Q.L.; Chan, P.; Yoshii, M.; Ueda, K. Alpha-synuclein aggregation and neurodegenerative diseases. J. Alzheimers Dis. 2003, 5, 139–148. [Google Scholar] [CrossRef]
- Meissner, W.G.; Frasier, M.; Gasser, T.; Goetz, C.G.; Lozano, A.; Piccini, P.; Obeso, J.A.; Rascol, O.; Schapira, A.; Voon, V.; et al. Priorities in Parkinson’s disease research. Nat. Rev. Drug Discov. 2011, 10, 377–393. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, D.H.; Block, M.L.; Lorenzl, S.; Yang, L.; Kim, Y.J.; Sugama, S.; Cho, B.P.; Hwang, O.; Browne, S.E.; et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007, 21, 179–187. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.S.; Cho, J.J.; Choi, D.H.; Hwang, O.; Shin, D.H.; Chun, H.S.; Beal, M.F.; Joh, T.H. Matrix metalloproteinase-3: A novel signaling proteinase from apoptotic neuronal cells that activates microglia. J. Neurosci. 2005, 25, 3701–3711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef] [PubMed]
- González, H.; Contreras, F.; Pacheco, R. Regulation of the Neurodegenerative Process Associated to Parkinson’s Disease by CD4+ T-cells. J. Neuroimmune Pharmacol. 2015, 10, 561–575. [Google Scholar] [CrossRef]
- Lucin, K.M.; Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron 2009, 64, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guajardo, V.; Tentillier, N.; Romero-Ramos, M. The relation between alpha-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience 2015, 302, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Kim, J.H.; Betarbet, R.; Greenamyre, J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003, 179, 9–16. [Google Scholar] [CrossRef]
- Lawrence, G.H.M. Taxonomy of Vascular Plants. Sci. Edu. 1952, 36, 311. [Google Scholar] [CrossRef]
- Ham, Y.M.; Yoon, W.J.; Park, S.Y.; Jung, Y.H.; Kim, D.; Jeon, Y.J.; Wijesinghe, W.A.; Kang, S.M.; Kim, K.N. Investigation of the component of Lycopodium serratum extract that inhibits proliferation and mediates apoptosis of human HL-60 leukemia cells. Food Chem. Toxicol. 2012, 50, 2629–2634. [Google Scholar] [CrossRef]
- Pathak, S.; Kumar Das, J.; Jyoti Biswas, S.; Khuda-Bukhsh, A.R. Protective potentials of a potentized homeopathic drug, Lycopodium-30, in ameliorating azo dye induced hepatocarcinogenesis in mice. Mol. Cell. Biochem. 2006, 285, 121–131. [Google Scholar] [CrossRef]
- Pathak, S.; Bhattacharjee, N.; Das, J.K.; Choudhury, S.C.; Karmakar, S.R.; Banerjee, P.; Paul, S.; Banerjee, A.; Khuda-Bukhsh, A.R. Supportive Evidence for the Anticancerous Potential of Alternative Medicine against Hepatocarcinogenesis in Mice. Complement. Med. Res. 2007, 14, 148–156. [Google Scholar] [CrossRef]
- Mandal, S.K.; Biswas, R.; Bhattacharyya, S.S.; Paul, S.; Dutta, S.; Pathak, S.; Khuda-Bukhsh, A.R. Lycopodine from Lycopodium clavatum extract inhibits proliferation of HeLa cells through induction of apoptosis via caspase-3 activation. Eur. J. Pharmacol. 2010, 626, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Paramita, P.; Subramaniam, V.D.; Murugesan, R.; Gopinath, M.; Ramachandran, I.; Ramalingam, S.; Sun, X.F.; Banerjee, A.; Marotta, F.; Pathak, S. Evaluation of potential anti-cancer activity of cationic liposomal nanoformulated Lycopodium clavatum in colon cancer cells. IET Nanobiotechnol. 2018, 12, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chandler, R.F.; Hanson, A.W. Obscurinine: A new lycopodium alkaloid. Tetrahedron Lett. 1987, 28, 5993–5996. [Google Scholar] [CrossRef]
- Brustolin Aleixo, C.F.; Ferraz, F.N.; Massini, P.F.; Lopes, C.R.; Falkowski Temporini, G.J.; Aleixo, D.L.; de Araújo, S.M. Beneficial immunomodulatory and neuro digestive effect in Trypanosoma cruzi infection after Lycopodium clavatum 13c treatment. Microb. Pathog. 2017, 112, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Henrique da Silva, G.; Barros, P.P.; Silva Goncalves, G.M.; Landi, M.A. Hepatoprotective effect of Lycopodium clavatum 30CH on experimental model of paracetamol-induced liver damage in rats. Homeopathy 2015, 104, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kupeli, E.; Sener, B.; Yesilada, E. Appraisal of anti-inflammatory potential of the clubmoss, Lycopodium clavatum L. J. Ethnopharmacol. 2007, 109, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gang, D.R. The Lycopodium alkaloids. Nat. Prod. Rep. 2004, 21, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Ellemann, L. Appendix to plants used by the Saraguros. Botanisk Institut Aarhus Universitet Aarhus 1990, 1, 1–46. [Google Scholar]
- Pedrosa, D.J.; Timmermann, L. Review: Management of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2013, 9, 321–340. [Google Scholar] [CrossRef]
- Pagano, G.; Rengo, G.; Pasqualetti, G.; Femminella, G.D.; Monzani, F.; Ferrara, N.; Tagliati, M. Cholinesterase inhibitors for Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Neuro. Psychiatr. 2015, 86, 767–773. [Google Scholar] [CrossRef]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and Therapeutic Strategies against Parkinson’s Disease: Recent Perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Elangovan, N.; Manigandan, K.; Singh, S.; Shukla, S. CNB-001 a novel curcumin derivative, guards dopamine neurons in MPTP model of Parkinson’s disease. Biomed. Res. Int. 2014, 2014, 236182. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Haque, M.E. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Devel. Ther. 2015, 9, 5499–5510. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Jain, P.D.; Sancheti, J.S.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology 2014, 86, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, Y.; Mitsui, C.; Uchiyama, N.; Hakamatsuka, T.; Morita, H. Hupercumines A and B, Lycopodium Alkaloids from Huperzia cunninghamioides, Inhibiting Acetylcholinesterase. Org. Lett. 2018, 20, 1384–1387. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Zhao, B.T.; Seong, S.H.; Kim, J.A.; Woo, M.H.; Choi, J.S.; Min, B.S. Inhibitory effects of serratene-type triterpenoids from Lycopodium complanatum on cholinesterases and beta-secretase 1. Chem. Biol. Interact. 2017, 274, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef]
- Hartmann, A. Postmortem studies in Parkinson’s disease. Dialogues Clin. Neurosci. 2004, 6, 281–293. [Google Scholar]
- Zecca, L.; Wilms, H.; Geick, S.; Claasen, J.H.; Brandenburg, L.O.; Holzknecht, C.; Panizza, M.L.; Zucca, F.A.; Deuschl, G.; Sievers, J.; et al. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: Implications for Parkinson’s disease. Acta Neuropathol. 2008, 116, 47–55. [Google Scholar] [CrossRef]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef]
- Ambani, L.M.; Van Woert, M.H.; Murphy, S. Brain peroxidase and catalase in parkinson disease. Arch. Neurol. 1975, 32, 114–118. [Google Scholar] [CrossRef]
- Konrath, E.L.; Neves, B.M.; Lunardi, P.S.; Passos Cdos, S.; Simoes-Pires, A.; Ortega, M.G.; Goncalves, C.A.; Cabrera, J.L.; Moreira, J.C.; Henriques, A.T. Investigation of the in vitro and ex vivo acetylcholinesterase and antioxidant activities of traditionally used Lycopodium species from South America on alkaloid extracts. J. Ethnopharmacol. 2012, 139, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanif, K.; Kumar, M.; Singh, N.; Shukla, R. Effect of homeopathic Lycopodium clavatum on memory functions and cerebral blood flow in memory-impaired rats. Homeopathy 2015, 104, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W. Metalloproteinases: Mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 2005, 6, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.M.; Lau, L.; Yong, V.W. MMPs in the central nervous system: Where the good guys go bad. Semin. Cell Dev. Biol. 2008, 19, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Jang, Y.J.; Kim, H.J.; Hwang, O. Tetrahydrobiopterin is released from and causes preferential death of catecholaminergic cells by oxidative stress. Mol. Pharmacol. 2000, 58, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, S.W.; Lee, S.Y.; Hwang, O. Dopamine-dependent cytotoxicity of tetrahydrobiopterin: A possible mechanism for selective neurodegeneration in Parkinson’s disease. J. Neurochem. 2003, 86, 143–152. [Google Scholar] [CrossRef]
- Sung, J.Y.; Park, S.M.; Lee, C.H.; Um, J.W.; Lee, H.J.; Kim, J.; Oh, Y.J.; Lee, S.T.; Paik, S.R.; Chung, K.C. Proteolytic cleavage of extracellular secreted α-synuclein via matrix metalloproteinases. J. Biol. Chem. 2005, 280, 25216–25224. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, Y.J.; Kim, Y.G.; Joh, T.H.; Beal, M.F.; Kim, Y.S. Role of matrix metalloproteinase 3-mediated alpha-synuclein cleavage in dopaminergic cell death. J. Biol. Chem. 2011, 286, 14168–14177. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, E.M.; Son, H.J.; Joh, T.H.; Kim, Y.S.; Kim, D.; Flint Beal, M.; Hwang, O. A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J. Neurochem. 2008, 106, 405–415. [Google Scholar] [CrossRef]
- Kim, E.M.; Hwang, O. Role of matrix metalloproteinase-3 in neurodegeneration. J. Neurochem. 2011, 116, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, S.; Calingasan, N.; Yang, L.; Albers, D.S.; Shugama, S.; Gregorio, J.; Krell, H.W.; Chirichigno, J.; Joh, T.; Beal, M.F. Matrix metalloproteinase-9 is elevated in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuromol. Med. 2004, 5, 119–132. [Google Scholar] [CrossRef]
- Annese, V.; Herrero, M.T.; Di Pentima, M.; Gomez, A.; Lombardi, L.; Ros, C.M.; De Pablos, V.; Fernandez-Villalba, E.; De Stefano, M.E. Metalloproteinase-9 contributes to inflammatory glia activation and nigro-striatal pathway degeneration in both mouse and monkey models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. Brain Struct. Funct. 2015, 220, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Hartlage-Rubsamen, M.; Lemke, R.; Schliebs, R. Interleukin-1beta, inducible nitric oxide synthase, and nuclear factor-kappaB are induced in morphologically distinct microglia after rat hippocampal lipopolysaccharide/interferon-gamma injection. J. Neurosci. Res. 1999, 57, 388–398. [Google Scholar] [CrossRef]
- Brown, G.C.; Bal-Price, A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol. Neurobiol. 2003, 27, 325–355. [Google Scholar] [CrossRef]
- Bal-Price, A.; Brown, G.C. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001, 21, 6480–6491. [Google Scholar] [CrossRef]
- Thakur, P.; Nehru, B. Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson’s disease. Neuroscience 2013, 231, 420–431. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Elangovan, N. In silico identification of potent inhibitors of alpha-synuclein aggregation and its in vivo evaluation using MPTP induced Parkinson mice model. Biomed. Aging Pathol. 2014, 4, 147–152. [Google Scholar] [CrossRef]
- Esteves, A.R.; Arduino, D.M.; Swerdlow, R.H.; Oliveira, C.R.; Cardoso, S.M. Oxidative stress involvement in alpha-synuclein oligomerization in Parkinson’s disease cybrids. Antioxid. Redox Signal. 2009, 11, 439–448. [Google Scholar] [CrossRef]
- Witt, S.N.; Flower, T.R. alpha-Synuclein, oxidative stress and apoptosis from the perspective of a yeast model of Parkinson’s disease. FEMS Yeast Res. 2006, 6, 1107–1116. [Google Scholar] [CrossRef]
- Xu, J.; Kao, S.Y.; Lee, F.J.; Song, W.; Jin, L.W.; Yankner, B.A. Dopamine-dependent neurotoxicity of alpha-synuclein: A mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002, 8, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Sala, G.; Arosio, A.; Stefanoni, G.; Melchionda, L.; Riva, C.; Marinig, D.; Brighina, L.; Ferrarese, C. Rotenone Upregulates Alpha-Synuclein and Myocyte Enhancer Factor 2D Independently from Lysosomal Degradation Inhibition. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Tabrizi, S.J.; Schapira, A.H.; Cooper, J.M. Alpha-synuclein expression in HEK293 cells enhances the mitochondrial sensitivity to rotenone. Neurosci. Lett. 2003, 351, 29–32. [Google Scholar] [CrossRef]
- Kilpatrick, K.; Novoa, J.A.; Hancock, T.; Guerriero, C.J.; Wipf, P.; Brodsky, J.L.; Segatori, L. Chemical induction of Hsp70 reduces alpha-synuclein aggregation in neuroglioma cells. ACS Chem. Biol. 2013, 8, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Nehru, B. Long-term heat shock proteins (HSPs) induction by carbenoxolone improves hallmark features of Parkinson’s disease in a rotenone-based model. Neuropharmacology 2014, 79, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Zeng, Y. Overview of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2012, 11, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Haavik, J.; Toska, K. Tyrosine hydroxylase and Parkinson’s disease. Mol. Neurobiol. 1998, 16, 285–309. [Google Scholar] [CrossRef]
- Emmrich, J.V.; Hornik, T.C.; Neher, J.J.; Brown, G.C. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 2013, 280, 5030–5038. [Google Scholar] [CrossRef]
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science 2002, 297, 1186–1190. [Google Scholar] [CrossRef]
- Kim, W.G.; Mohney, R.P.; Wilson, B.; Jeohn, G.H.; Liu, B.; Hong, J.S. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J. Neurosci. 2000, 20, 6309–6316. [Google Scholar] [CrossRef]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Meeran, M.F.N.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson’s Disease. Molecules 2019, 24, 2182. https://doi.org/10.3390/molecules24112182
Jayaraj RL, Beiram R, Azimullah S, Meeran MFN, Ojha SK, Adem A, Jalal FY. Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson’s Disease. Molecules. 2019; 24(11):2182. https://doi.org/10.3390/molecules24112182
Chicago/Turabian StyleJayaraj, Richard L., Rami Beiram, Sheikh Azimullah, Mohamed Fizur Nagoor Meeran, Shreesh K. Ojha, Abdu Adem, and Fakhreya Yousuf Jalal. 2019. "Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson’s Disease" Molecules 24, no. 11: 2182. https://doi.org/10.3390/molecules24112182
APA StyleJayaraj, R. L., Beiram, R., Azimullah, S., Meeran, M. F. N., Ojha, S. K., Adem, A., & Jalal, F. Y. (2019). Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson’s Disease. Molecules, 24(11), 2182. https://doi.org/10.3390/molecules24112182