2-Iodo-4′-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway
Abstract
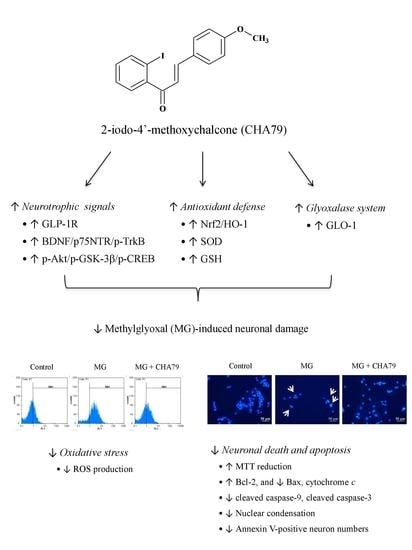
:1. Introduction
2. Results
2.1. CHA79 Mitigates Neuronal Apoptosis Accompany with Modulation of Anti-Apoptotic and Apoptotic Signals in MG-Treated SH-SY5Y Dopaminergic Neurons
2.2. CHA79 Enhances GLP-1R, BDNF, and Related Neurotrophic Signals in MG-Treated SH-SY5Y Dopaminergic Neurons
2.3. CHA79 Relieves Oxidative Stress via Regulation of Antioxidant Defense and Glyoxalase Pathway in MG-Treated SH-SY5Y Dopaminergic Neurons
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Cultures and Procedure of Drugs Management
4.3. Evaluation of Cell Viability and Neuronal Apoptosis
4.4. Evaluation of Oxidative Stress
4.5. Evaluation of Antioxidant Defense
4.6. Western Blots (WB)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [PubMed]
- Huang, X.; Wang, F.; Chen, W.; Chen, Y.; Wang, N.; von Maltzan, K. Possible link between the cognitive dysfunction associated with diabetes mellitus and the neurotoxicity of methylglyoxal. Brain Res. 2012, 1469, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Y.; Nodera, H.; Osaki, Y.; Banzrai, C.; Takayasu, K.; Endo, S.; Shibuta, Y.; Kaji, R. Upregulation of axonal HCN current by methylglyoxal: Potential association with diabetic polyneuropathy. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Biosa, A.; Outeiro, T.F.; Bubacco, L.; Bisaglia, M. Diabetes Mellitus as a Risk Factor for Parkinson’s Disease: A Molecular Point of View. Mol. Neurobiol. 2018, 55, 8754–8763. [Google Scholar] [CrossRef] [PubMed]
- Amicarelli, F.; Colafarina, S.; Cattani, F.; Cimini, A.; Di Ilio, C.; Ceru, M.P.; Miranda, M. Scavenging system efficiency is crucial for cell resistance to ROS-mediated methylglyoxal injury. Free Radic. Biol. Med. 2003, 35, 856–871. [Google Scholar] [CrossRef]
- Desai, K.M.; Wu, L. Free radical generation by methylglyoxal in tissues. Drug Metab. Drug Int. 2008, 23, 151–173. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Peres, A.; Ferreira, G.C. Pinocembrin Attenuates Mitochondrial Dysfunction in Human Neuroblastoma SH-SY5Y Cells Exposed to Methylglyoxal: Role for the Erk1/2-Nrf2 Signaling Pathway. Neurochem. Res. 2017, 42, 1057–1072. [Google Scholar] [CrossRef]
- Nishimoto, S.; Koike, S.; Inoue, N.; Suzuki, T.; Ogasawara, Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: Increase in GSH levels is a critical event for the detoxification mechanism. Biochem. Biophys. Res. Commun. 2017, 483, 874–879. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Methylglyoxal-induced dicarbonyl stress in aging and disease: First steps towards glyoxalase 1-based treatments. Clin. Sci. 2016, 130, 1677–1696. [Google Scholar] [CrossRef]
- Sharma, M.K.; Jalewa, J.; Holscher, C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J. Neurochem. 2014, 128, 459–471. [Google Scholar] [CrossRef]
- Di Loreto, S.; Zimmitti, V.; Sebastiani, P.; Cervelli, C.; Falone, S.; Amicarelli, F. Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int. J. Biochem. Cell Biol. 2008, 40, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S. Jr.; Castro, A.A.; Moreira, E.L.; Glaser, V.; Santos, A.R.; Tasca, C.I.; Latini, A.; Prediger, R.D. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 2011, 132, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.N.; Florang, V.R.; Eckert, L.L.; Doorn, J.A. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem. Res. Toxicol. 2009, 22, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Hsieh, T.J.; El-Shazly, M.; Chuang, D.W.; Tsai, Y.H.; Yen, C.T.; Wu, S.F.; Wu, Y.C.; Chang, F.R. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg. Med. Chem. Lett. 2012, 22, 3912–3915. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Chang, F.R.; Tsai, Y.H.; Wu, Y.C.; Hsieh, T.J. 2-Bromo-4′-methoxychalcone and 2-Iodo-4′-methoxychalcone Prevent Progression of Hyperglycemia and Obesity via 5′-Adenosine-Monophosphate-Activated Protein Kinase in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 2763. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Ötvös, S.B.; Wu, Y.-C.; Mándity, I.M.; Chang, F.-R.; Fülöp, F. Highly Selective Continuous-Flow Synthesis of Potentially Bioactive Deuterated Chalcone Derivatives. ChemPlusChem 2015, 80, 859–864. [Google Scholar] [CrossRef]
- Maessen, D.E.; Stehouwer, C.D.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Matafome, P.; Sena, C.; Seica, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Stehouwer, C.D.; Schalkwijk, C.G. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem. Soc. Trans. 2014, 42, 443–449. [Google Scholar] [CrossRef]
- Srikanth, V.; Westcott, B.; Forbes, J.; Phan, T.G.; Beare, R.; Venn, A.; Pearson, S.; Greenaway, T.; Parameswaran, V.; Munch, G. Methylglyoxal, cognitive function and cerebral atrophy in older people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Vicente Miranda, H.; Szego, E.M.; Oliveira, L.M.A.; Breda, C.; Darendelioglu, E.; de Oliveira, R.M.; Ferreira, D.G.; Gomes, M.A.; Rott, R.; Oliveira, M.; et al. Glycation potentiates alpha-synuclein-associated neurodegeneration in synucleinopathies. Brain A J. Neurol. 2017, 140, 1399–1419. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Du, L.L.; Cheng, X.S.; Jiang, X.; Zhang, Y.; Lv, B.L.; Liu, R.; Wang, J.Z.; Zhou, X.W. Glycation exacerbates the neuronal toxicity of beta-amyloid. Cell Death Dis. 2013, 4, e673. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Leo, A.; Andreozzi, F.; Citraro, R.; Iannone, M.; Spiga, R.; Sesti, G.; Constanti, A.; De Sarro, G.; Arturi, F.; et al. Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav. Brain Res. 2017, 321, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Perry, T.; Kindy, M.S.; Harvey, B.K.; Tweedie, D.; Holloway, H.W.; Powers, K.; Shen, H.; Egan, J.M.; Sambamurti, K.; et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Nat. Acad. Sci. USA 2009, 106, 1285–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, Y.T.; Lin, W.J.; Chang, W.H.; Lo, Y.C. The novel protective effects of loganin against 1-methyl-4-phenylpyridinium-induced neurotoxicity: Enhancement of neurotrophic signaling, activation of IGF-1R/GLP-1R, and inhibition of RhoA/ROCK pathway. Phytother. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Choi, H.I.; Wang, Y.; Luo, Y.; Hoffer, B.J.; Greig, N.H. A New Treatment Strategy for Parkinson’s Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway. Cell Trans. 2017, 26, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Barker, R.A. Neurotrophic factors as a therapeutic target for Parkinson’s disease. Expert Opin. Ther. Targets 2008, 12, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Lu, J.J.; Zhou, X.F.; Wang, Y.J. Roles of p75NTR in the pathogenesis of Alzheimer’s disease: A novel therapeutic target. Biochem. Pharmacol. 2011, 82, 1500–1509. [Google Scholar] [CrossRef]
- Fujii, T.; Kunugi, H. p75NTR as a therapeutic target for neuropsychiatric diseases. Curr. Mol. Pharmacol. 2009, 2, 70–76. [Google Scholar] [CrossRef]
- Kanning, K.C.; Hudson, M.; Amieux, P.S.; Wiley, J.C.; Bothwell, M.; Schecterson, L.C. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 5425–5436. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Ann. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, S.; Zhou, X.; Li, Y.; Bai, J. Brain-derived neurotrophic factor induces thioredoxin-1 expression through TrkB/Akt/CREB pathway in SH-SY5Y cells. Biochimie 2019, 160, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Liang, Z.; Wang, T.T.; Yang, J.W.; Ma, W.; Deng, S.K.; Wang, X.B.; Dai, Y.F.; Guo, J.H.; Li, L.Y. Brain-derived Neurotrophic Factor Promotes Growth of Neurons and Neural Stem Cells Possibly by Triggering the Phosphoinositide 3-Kinase/ AKT/Glycogen Synthase Kinase-3beta/beta-catenin Pathway. CNS Neurol. Disord. Drug Targets 2017, 16, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Chen, C.S.; Jong, Y.J.; Chang, F.R.; Lo, Y.C. Loganin possesses neuroprotective properties, restores SMN protein and activates protein synthesis positive regulator Akt/mTOR in experimental models of spinal muscular atrophy. Pharmacol. Res. 2016, 111, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.R.; Narayanasamy, P. Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway. Redox Biol. 2018, 14, 465–473. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Archiv. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Chen, C.S.; Tseng, Y.T.; Hsu, Y.Y.; Lo, Y.C. Nrf2-Keap1 antioxidant defense and cell survival signaling are upregulated by 17beta-estradiol in homocysteine-treated dopaminergic SH-SY5Y cells. Neuroendocrinology 2013, 97, 232–241. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Tseng, Y.T.; Lo, Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Hsu, Y.Y.; Shih, Y.T.; Lo, Y.C. Paeonol attenuates microglia-mediated inflammation and oxidative stress-induced neurotoxicity in rat primary microglia and cortical neurons. Shock 2012, 37, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Chang, F.R.; Lo, Y.C. The Chinese herbal formula Liuwei dihuang protects dopaminergic neurons against Parkinson’s toxin through enhancing antioxidative defense and preventing apoptotic death. Phytomed. Int. J. Phytother. Phytopharmacol. 2014, 21, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Shih, Y.T.; Tseng, Y.T.; Hsu, H.T. Neuroprotective Effects of San-Huang-Xie-Xin-Tang in the MPP(+)/MPTP Models of Parkinson’s Disease In Vitro and In Vivo. Evid. Based Complement Altern. Med. 2012, 2012, 501032. [Google Scholar]
- Bahar, E.; Lee, G.H.; Bhattarai, K.R.; Lee, H.Y.; Choi, M.K.; Rashid, H.O.; Kim, J.Y.; Chae, H.J.; Yoon, H. Polyphenolic Extract of Euphorbia supina Attenuates Manganese-Induced Neurotoxicity by Enhancing Antioxidant Activity through Regulation of ER Stress and ER Stress-Mediated Apoptosis. Int. J. Mol. Sci. 2017, 18, 300. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.; Narayanasamy, P. Flavonoid Enhances the Glyoxalase Pathway in Cerebellar Neurons to Retain Cellular Functions. Sci. Rep. 2017, 7, 5126. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. Methylglyoxal, advanced glycation end products and autism: Is there a connection? Med. Hypotheses 2012, 78, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Zhu, X.; Zhang, L.; Lu, Q.; Wang, J.Y.; Zhang, F.; Guo, H.; Yin, J.L.; Yin, X.X. Up-regulation of glyoxalase 1 by mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2013, 721, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Zhu, X.; Zhang, L.; Lu, Q.; Zhang, F.; Guo, H.; Yin, X.X. Cerebroprotective effects of ibuprofen on diabetic encephalopathy in rats. Pharmacol. Biochem. Behav. 2014, 117, 128–136. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Rad. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, M.R.; Ferreira, G.C.; Schuck, P.F.; Dal Bosco, S.M. Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem. Biol. Interact. 2015, 242, 396–406. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Rizzo, B.; Barbalace, M.C.; Fabbri, D.; Hrelia, S. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem. Res. Toxicol. 2015, 28, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds not available are available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Y.-T.; Tsai, Y.-H.; Fülöp, F.; Chang, F.-R.; Lo, Y.-C. 2-Iodo-4′-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway. Molecules 2019, 24, 2249. https://doi.org/10.3390/molecules24122249
Tseng Y-T, Tsai Y-H, Fülöp F, Chang F-R, Lo Y-C. 2-Iodo-4′-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway. Molecules. 2019; 24(12):2249. https://doi.org/10.3390/molecules24122249
Chicago/Turabian StyleTseng, Yu-Ting, Yi-Hong Tsai, Ferenc Fülöp, Fang-Rong Chang, and Yi-Ching Lo. 2019. "2-Iodo-4′-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway" Molecules 24, no. 12: 2249. https://doi.org/10.3390/molecules24122249
APA StyleTseng, Y. -T., Tsai, Y. -H., Fülöp, F., Chang, F. -R., & Lo, Y. -C. (2019). 2-Iodo-4′-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway. Molecules, 24(12), 2249. https://doi.org/10.3390/molecules24122249