A Membrane-Based Process for the Recovery of Glycyrrhizin and Phenolic Compounds from Licorice Wastewaters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Clarification of Licorice Wastewater
2.2. UF and NF of Clarified Licorice Wastewaters: Flux Evaluation
2.3. UF and NF of Clarified Licorice Wastewaters: Analyses of Membrane Selectivity
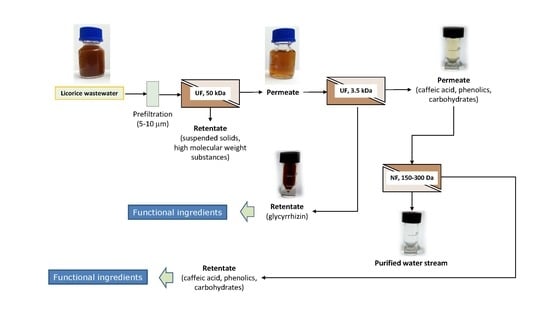
2.4. Integrated Membrane Process
3. Materials and Methods
3.1. Licorice Aqueous Solutions
3.2. Clarification of Licorice Wastewater
3.3. Fractionation of Clarified Solution with UF and NF Membranes: Set-Up and Procedures
3.4. Analytical Measurements
3.4.1. Determination of Suspended Solids
3.4.2. Determination of Total Polyphenols
3.4.3. Determination of Glicyrrhizic Acid and Caffeic Acid
3.4.4. Determination of Total Antioxidant Activity
3.4.5. Determination of Total Carbohydrates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Obolentseva, G.V.; Litvinenko, V.I.; Ammosov, A.S.; Popova, T.P.; Sampiev, A.M. Pharmacological and therapeutic properties of licorice preparations. Pharm. Chem. J. 1999, 33, 24–31. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef]
- Yin, G.; Cao, L.; Xu, P.; Jeney, G.; Nakao, M.; Lu, C. Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against carbon tetrachloride (CCl4)-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol. Biochem. 2011, 37, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljević, S.; Šibul, F.; Sinka, I.; Zupko, I.; Ocsovszki, I.; Jovanović-Šanta, S. Chemical composition, antioxidant and anticancer activity of licorice from Fruska Gora locality. Ind. Crop. Prod. 2018, 112, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Tong, Z.; Tong, P.; Sun, J. Pilot study on treatment process of licorice products wastewater. China Water Wast. 2010, 26, 58–62. [Google Scholar]
- Ramaswami, S.; Behrendt, J.; Gulyas, H.; Otterpohl, R. Pretreatment of wastewater from licorice processing—A preliminary evaluation. Water Environ. Res. 2016, 88, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yang, L.C.; Poe, N.; Huang, H.B. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Separation of glycyrrhizic acid from licorice root extract using macroporous resin. Food Bioprod. Process. 2015, 93, 51–57. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Extraction of glycyrrhizic acid from licorice root using ultrasound: Process intensification studies. Chem. Eng. Process. 2012, 54, 37–41. [Google Scholar] [CrossRef]
- Pan, X.; Liu, H.; Jia, G.; Shu, Y.Y. Microwave-assisted extraction of glycyrrhizic acid from licorice root. Biochem. Eng. J. 2000, 5, 173–177. [Google Scholar] [CrossRef]
- Hedayati, A.; Ghoreishi, S.M. Supercritical carbon dioxide extraction of glycyrrhizic acid from licorice plant root using binary entrainer: Experimental optimization via response surface methodology. J. Supercrit. Fluids 2015, 100, 209–217. [Google Scholar] [CrossRef]
- Jiang, Y.; Lua, H.; Chen, F. Preparative purification of glycyrrhizin extracted from the root of liquorice using high-speed counter-current chromatography. J. Chromatogr. A 2004, 1033, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, J.; Kong, L. Simultaneous separation of triterpenoid saponins and flavonoid glycosides from the roots of Glycyrrhiza uralensis Fisch by pH-zone-refining counter-current chromatography. J. Sep. Sci. 2013, 36, 3295–3301. [Google Scholar] [PubMed]
- Nath, K.; Dave, H.K.; Patel, T.M. Revisiting the recent applications of nanofiltration in food processing industries: progress and prognosis. Trends Food Sci. Technol. 2018, 73, 12–24. [Google Scholar] [CrossRef]
- Tylkowski, B.; Nowak, M.; Tsibranska, I.; Trojanowska, A.; Marciniak, L.; Garcia Valls, R.; Gumi, T.; Giamberini, M.; Jastrzab, R. Concentration and fractionation of polyphenols by membrane operations. Curr. Pharm. Design 2017, 23, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Bagci, P.O. Production of phytotherapeutics from broccoli juice by integrated membrane processes. Food Chem. 2018, 242, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Madaeni, S.S.; Sohrabi, M.R.; Khosravi, M.; Ghaedi, A.M. Separation of liquiritin from glycyrrhizic acid in licorice root extract by nanofiltration membrane. Sep. Sci. Technol. 2011, 46, 1406–1413. [Google Scholar] [CrossRef]
- Gorgol, L.G.; Taub, S.G.; Olesiewicz, J. Separation of glycyrrhizic acid from licorice extract by ultrafiltration. U.S. Patent 0,196,138, 11 August 2011. [Google Scholar]
- Chhaya; Mondal, S.; Majumdar, G.C.; De, S. Clarifications of stevia extract using cross flow ultrafiltration and concentration by nanofiltration. Sep. Purif. Technol. 2012, 89, 125–134. [Google Scholar] [CrossRef]
- Hashemi, P.; Beyranvand, S.; Mansur, R.S.; Ghiasvand, A.R. Development of a simple device for dispersive liquid-liquid microextraction with lighter than water organic solvents: isolation and enrichment of glycyrrhizic acid from licorice. Anal. Chim. Acta 2009, 655, 60–65. [Google Scholar] [CrossRef]
- Baek, J.Y.; Lee, J.M.; Lee, S.C. Extraction of nutraceutical compounds from licorice roots with subcritical water. Sep. Purif. Technol. 2008, 63, 661–664. [Google Scholar] [CrossRef]
- Benitez, F.J.; Acero, J.L.; Real, F.J.; Roldán, G.; Rodriguez, E. Ultrafiltration and nanofiltration membranes applied to the removal of the pharmaceuticals amoxicillin, naproxen, metoprolol and phenacetin from water. J. Chem. Technol. Biotechnol. 2011, 86, 858–866. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajό, J.C. Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Cissé, M.; Vaillant, F.; Pallet, D.; Dornier, M. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res. Int. 2011, 44, 2607–2614. [Google Scholar] [CrossRef]
- Cassano, A.; Cabri, W.; Mombelli, G.; Peterlongo, F.; Giorno, L. Recovery of bioactive compounds from artichoke brine by nanofiltration. Food Bioprod. Process. 2016, 98, 257–265. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Madaeni, S.S.; Khosravi, M.; Ghaedi, A.M. Chemical cleaning of reverse osmosis and nanofiltration membranes fouled by licorice aqueous solutions. Desalination 2011, 267, 93–100. [Google Scholar] [CrossRef]
- Brazinha, C.; Cadima, M.; Crespo, J.G. Valorisation of spent coffee through membrane processing. J. Food Eng. 2015, 149, 123–130. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Madaeni, S.S.; Khosravi, M.; Ghaedi, A.M. Concentration of licorice aqueous solutions using nanofiltration and reverse osmosis membranes. Sep. Purif. Technol. 2010, 75, 121–126. [Google Scholar] [CrossRef]
- Sun, C.; Xie, Y.; Tian, Q.; Liu, H. Separation of glycyrrhizic acid and liquiritin from licorice root by aqueous nonionic surfactant mediated extraction. Colloid Surf. A Physicochem. Eng. Asp. 2007, 305, 42–47. [Google Scholar] [CrossRef]
- Tres, M.V.; Helen, C.; Ferraz, H.C.; Dallogo, R.M.; Di Luccio, M.; Oliveria, J.V. Characterization of polymeric membranes used in vegetable oil/organic solvents separation. J. Membr. Sci. 2010, 362, 495–500. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Acosta, O.; Vaillant, F.; Pérez, A.M.; Dornier, M. Potential of ultrafiltration for separation and purification of ellagitannins in blackberry (Rubus adenotrichus Schltdl.) juice. Sep. Purif. Technol. 2014, 125, 120–125. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. A membrane-based study for the recovery of polyphenols from bergamot juice. J. Membr. Sci. 2011, 375, 182–190. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; De Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Negrão Murakami, A.N.; de Mello Castanho Amboni, R.D.; Prudêncio, E.S.; Amante, E.R.; Fritzen-Freire, C.B.; Bremer Boaventura, B.C.; Muñoz, I.D.; Branco, C.D.; Salvador, M.; Maraschin, M. Concentration of biologically active compounds extracted from Ilex paraguariensis St. Hil. by nanofiltration. Food Chem. 2011, 141, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajό, J.C. Membrane concentration of antioxidants from Castanea sativa leaves aqueous extracts. Chem. Eng. J. 2011, 175, 95–102. [Google Scholar] [CrossRef]
- Vieira, G.S.; Moreira, F.K.V.; Matsumoto, R.L.S.; Michelon, M.; Filho, F.M.; Hubinger, M.D. Influence of nanofiltration membrane features on enrichment of jussara ethanolic extract (Euterpe edulis) in anthocyanins. J. Food Eng. 2018, 226, 31–41. [Google Scholar] [CrossRef]
- Bowen, W.R.; Doneva, T.A. Atomic force microscopy characterization of ultrafiltration membranes: correspondence between surface pore dimensions and molecular weight cut-off. Surf. Interface Anal. 2000, 29, 544–547. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available. |







| Parameter | Feed | Permeate | Retentate |
|---|---|---|---|
| Suspended solids (%, w/w) | 1.9 ± 0.1 | n.d. | 5.6 ± 0.2 |
| Total carbohydrates (g/L) | 5.03 ± 0.01 | 4.24 ± 0.03 | 8.11 ± 0.01 |
| Glycyrrhizic acid (mg/L) | 224.3 ± 4.5 | 210.1 ± 4.20 | 256.35 ± 5.13 |
| Caffeic acid (mg/L) | 27.1 ± 0.5 | 24.3 ± 0.5 | 28.1 ± 0.6 |
| TAA (mM Trolox) | 6.0 ± 0.6 | 5.9 ± 2.8 | 6.2 ± 0.7 |
| Total polyphenols (mg/L gallic acid) | 883.7 ± 30.7 | 844.9 ± 26.2 | 1165.9 ± 8.8 |
| Membrane Type | ||||
|---|---|---|---|---|
| GK | GH | GE | DK | |
| FDR (%) | 50 | 35 | 20 | 47 |
| FI (%) | 34.03 | 19.45 | 22.03 | 27.25 |
| CE (%) | 91.50 | 92 | 98.23 | 100 |
| Membrane Type | Sample | WRF | Total Polyphenols (mg/L) | Total Carbohydrates (mg/L) | Caffeic Acid (mg/L) | Glycyrrhizin (mg/L) | TAA (mM Trolox) |
|---|---|---|---|---|---|---|---|
| GK | Feed | 764.5 ± 12.3 | 3.99 ± 0.03 | 22.1 ± 0.4 | 207.3 ± 4.1 | 5.1 ± 0.4 | |
| Permeate | 2 | 604.6 ± 6.1 | 2.86 ± 0.03 | 21.8 ± 0.4 | n.d. | 3.7 ± 0.4 | |
| 3 | 630.6 ± 37.0 | 2.89 ± 0.01 | 22.1 ± 0.4 | n.d. | 3.6 ± 0.5 | ||
| 4 | 595.9 ± 20.5 | 2.93 ± 0.01 | 22.1 ± 0.4 | n.d. | 3.4 ± 0.3 | ||
| 5 | 587.2 ± 5.8 | 2.99 ± 0.02 | 22.1 ± 0.4 | n.d. | 3.3 ± 0.1 | ||
| Retentate | 2 | 1017.4 ± 32.9 | 4.41 ± 0.02 | 27.1 ± 0.5 | 377.1 ± 7.5 | 7.4 ± 0.2 | |
| 3 | 1186.1 ± 49.3 | 5.53 ± 0.02 | 35.1 ± 0.7 | 578.3 ± 11.5 | 8.4 ± 0.3 | ||
| 4 | 1337.2 ± 8.2 | 7.32 ± 0.02 | 42.3 ± 0.8 | 755.7 ± 15.1 | 9.1 ± 0.5 | ||
| 5 | 1395.3 ± 8.2 | 8.10 ± 0.01 | 54.1 ± 1.1 | 863.6 ± 17.3 | 10.5 ± 0.4 | ||
| GH | Feed | 746.1 ± 3.3 | 4.12 ± 0.03 | 20.5 ± 0.4 | 192.5 ± 3.8 | 5.0 ± 0.5 | |
| Permeate | 2 | 523.2 ± 5.8 | 2.40 ± 0.01 | 19.5 ± 0.2 | n.d. | 3.6 ± 0.4 | |
| 3 | 534.8 ± 5.8 | 2.43 ± 0.01 | 20.1 ± 0.4 | n.d. | 3.3 ± 0.1 | ||
| 4 | 536.8 ± 6.7 | 2.52 ± 0.01 | 19.8 ± 0.4 | n.d. | 3.0 ± 0.4 | ||
| 5 | 529.1 ± 5.8 | 2.61 ± 0.02 | 20.1 ± 0.4 | n.d. | 2.8 ± 0.3 | ||
| Retentate | 2 | 1127.9 ± 5.8 | 4.49 ± 0.04 | 35.6 ± 0.7 | 282.0 ± 5.6 | 6.9 ± 0.9 | |
| 3 | 1246.1 ± 12.1 | 5.70 ± 0.03 | 50.8 ± 1.0 | 412.0 ± 8.2 | 8.0 ± 0.7 | ||
| 4 | 1492.2 ± 12.1 | 8.11 ± 0.01 | 59.4 ± 1.2 | 768.0 ± 15.3 | 10.0 ± 0.7 | ||
| 5 | 1908.9 ± 8.8 | 9.05 ± 0.06 | 89.1 ± 1.8 | 900.6 ± 18.0 | 13.6 ± 0.5 | ||
| GE | Feed | 775.2 ± 3.3 | 4.10 ± 0.05 | 20.7 ± 0.4 | 173.9 ± 3.5 | 5.3 ± 0.4 | |
| Permeate | 2 | 434.1 ± 3.3 | 2.08 ± 0.01 | 18.1 ± 0.3 | n.d. | 3.0 ± 0.3 | |
| 3 | 395.3 ± 10.1 | 2.10 ± 0.01 | 17.1 ± 0.3 | n.d. | 2.6 ± 0.3 | ||
| 4 | 374.0 ± 6.7 | 2.14 ± 0.02 | 17.3 ± 0.3 | n.d. | 2.3 ± 0.5 | ||
| 5 | 407.0 ± 4.1 | 2.17 ± 0.02 | 18.3 ± 0.3 | n.d. | 2.6 ± 0.4 | ||
| Retentate | 2 | 1129.8 ± 23.5 | 5.30 ± 0.03 | 36.1 ± 0.7 | 316.7 ± 6.3 | 7.4 ± 0.6 | |
| 3 | 1455.4 ± 16.8 | 6.73 ± 0.03 | 47.5 ± 0.9 | 485.1 ± 9.7 | 9.3 ± 3.3 | ||
| 4 | 1839.1 ± 18.7 | 8.61 ± 0.46 | 79.9 ± 1.6 | 642.9 ± 12.9 | 9.6 ± 0.1 | ||
| 5 | 2071.7 ± 13.4 | 9.21 ± 0.06 | 94.5 ± 1.9 | 792.1 ± 15.8 | 12.8 ± 0.8 | ||
| DK | Feed | 799.4 ± 20.5 | 4.08 ± 0.02 | 19.3 ± 0.4 | 208.2 ± 4.2 | 4.9 ± 0.3 | |
| Permeate | 2 | 319.7 ± 8.2 | 1.64 ± 0.02 | 11.4 ± 0.2 | n.d. | 1.7 ± 0.4 | |
| 3 | 308.1 ± 3.1 | 1.64 ± 0.03 | 12.3 ± 0.2 | n.d. | 1.5 ± 0.4 | ||
| 4 | 305.2 ± 4.1 | 1.73 ± 0.02 | 13.1 ± 0.2 | n.d. | 1.5 ± 0.5 | ||
| 5 | 293.6 ± 4.1 | 1.75 ± 0.02 | 12.4 ± 0.2 | n.d. | 0.7 ± 0.5 | ||
| Retentate | 2 | 1180.2 ± 10.1 | 5.86 ± 0.01 | 32.1 ± 0.6 | 315.9 ± 6.3 | 9.6 ± 0.2 | |
| 3 | 1363.4 ± 12.3 | 7.55 ± 0.01 | 43.5 ± 0.8 | 534.9 ± 10.7 | 11.6 ± 0.3 | ||
| 4 | 1857.5 ± 20.5 | 9.13 ± 0.06 | 52.8 ± 1.1 | 823.9 ± 16.5 | 15.6 ± 0.9 | ||
| 5 | 2247.1 ± 20.5 | 10.11 ± 0.06 | 90.1 ± 1.8 | 1085.8 ± 21.7 | 21.2 ± 1.1 |
| Membrane Type | DK | GE | GH | GK |
|---|---|---|---|---|
| Manufacturer | GE Osmonics | GE Osmonics | GE Osmonics | GE Osmonics |
| Membrane material | PA-TFC | PA-TFC | PA-TFC | PA-TFC |
| Nominal MWCO (Da) | 150–300 | 1000 | 2500 | 3500 |
| pH operating range | 3–9 | 2–10 | 2–10 | 2–10 |
| pH range in cleaning conditions | 2–10.5 | 1–11.5 | 1–11.5 | 1–11.5 |
| Max. operating temperature (°C) | 50 | 50 | 50 | 50 |
| Max. operating pressure (bar) | 41 | 27.6 | 27.6 | 27.6 |
| Membrane surface area (m2) | 0.32 | 0.32 | 0.32 | 0.32 |
| Contact angle (°) | 37.9 ± 3.3 a | 73.0 ± 0.5 b | 62.0 ± 1.1 b | 71.0 ± 1.4 b |
| Average pore width (nm) | 9.6 a | - | - | - |
| Mean pore diameter (nm) | - | 1.83 ± 0.35 c | 2.23 ± 0.46 c | 2.52 ± 0.51 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conidi, C.; Fucà, L.; Drioli, E.; Cassano, A. A Membrane-Based Process for the Recovery of Glycyrrhizin and Phenolic Compounds from Licorice Wastewaters. Molecules 2019, 24, 2279. https://doi.org/10.3390/molecules24122279
Conidi C, Fucà L, Drioli E, Cassano A. A Membrane-Based Process for the Recovery of Glycyrrhizin and Phenolic Compounds from Licorice Wastewaters. Molecules. 2019; 24(12):2279. https://doi.org/10.3390/molecules24122279
Chicago/Turabian StyleConidi, Carmela, Lidia Fucà, Enrico Drioli, and Alfredo Cassano. 2019. "A Membrane-Based Process for the Recovery of Glycyrrhizin and Phenolic Compounds from Licorice Wastewaters" Molecules 24, no. 12: 2279. https://doi.org/10.3390/molecules24122279
APA StyleConidi, C., Fucà, L., Drioli, E., & Cassano, A. (2019). A Membrane-Based Process for the Recovery of Glycyrrhizin and Phenolic Compounds from Licorice Wastewaters. Molecules, 24(12), 2279. https://doi.org/10.3390/molecules24122279