The Antibiofilm Effect of a Medical Device Containing TIAB on Microorganisms Associated with Surgical Site Infection
Abstract
:1. Introduction
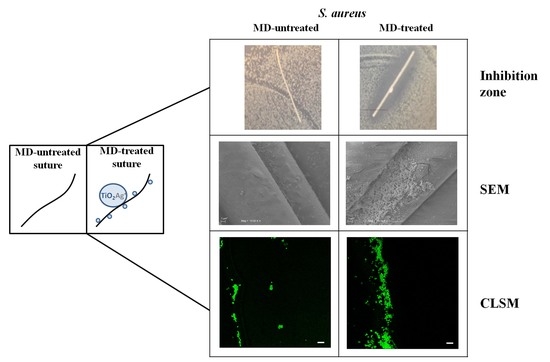
2. Results and Discussion
3. Materials and Methods
3.1. Bacterial Strains and Culture Conditions
3.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.3. Determination of Minimal Biofilm Inhibitory Concentration (MBIC)
3.4. Coating of the Sutures with the MD
3.5. Antimicrobial and Antibiofilm Activity of the MD-Treated Sutures
3.6. Scanning Electron Microscopy (SEM)
3.7. Confocal Laser Scanning Microscopy (CLSM) Analysis for Visualization of Adherent Bacteria
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect. Control Hosp. Epidemiol. 1992, 13, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Onesti, M.G.; Carella, S.; Scuderi, N. Effectiveness of antimicrobial-coated sutures for the prevention of surgical site infection: A review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5729–5739. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Drapeau, C.M.J.; Nicastri, E.; Martini, L.; Ippolito, G.; Moro, M.L.; ANIPIO. Surgical site infections in Italian Hospitals: A prospective multicenter study. BMC Infect. Dis. 2008, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Nistico, L.; Sambanthamoorthy, K.; Longwell, M.; Iannitelli, A.; Cellini, L.; Di Stefano, A.; Hall Stoodley, L.; Stoodley, P. Temporal expression of agrB, cidA, and alsS in the early development of Staphylococcus aureus UAMS-1 biofilm formation and the structural role of extracellular DNA and carbohydrates. Pathog. Dis. 2014, 70, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutics strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Purba, A.K.R.; Setiawan, D.; Bathoorn, E.; Postma, M.J.; Dik, J.H.; Friedrich, A.W. Prevention of surgical site infections: A systematic review of cost analyses in the use of prophylactic antibiotics. Front. Pharmacol. 2018, 9, 776. [Google Scholar] [CrossRef]
- Aytekin Aydın, M.T.; Hoşgün, H.L.; Dede, A.; Güven, K. Synthesis, characterization and antibacterial activity of silver-doped TiO2 nanotubes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 503–507. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Weng, S.; Zhao, X.; Liu, G.; Guan, Y.; Wu, F.; Luo, Y. Synthesis, characterization, antibacterial activity in dark and in vitro cytocompatibility of Ag-incorporated TiO2 microspheres with high specific surface area. J. Mater. Sci. Mater. Med. 2018, 29, 50. [Google Scholar] [CrossRef] [PubMed]
- Candotto, V.; Lauritano, D.; Carinci, F.; Bignozzi, C.A.; Pazzi, D.; Cura, F.; Severino, M.; Scarano, A. Silver-based chemical device as an adjunct of domestic oral hygiene: A study on periodontal patients. Materials (Basel) 2018, 11, 1483. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kroon, R.E.; Craciun, V.; Popa, M.; Chifiriuc, M.C.; Swart, H.C. Influence of Ag, Au and Pd noble metals doping on structural, optical and antimicrobial properties of zinc oxide and titanium dioxide nanomaterials. Heliyon 2019, 5, e01333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiris, M.; Gunasekara, T.; Jayaweera, P.M.; Fernando, S. TiO2 nanoparticles from Baker’s yeast: A potent antimicrobial. J. Microbiol. Biotechnol. 2018, 28, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Namratha, K.; Alghamdi, S.; Mohammad, Y.H.E.; Hezam, A.; Zare, M.; Drmosh, Q.A.; Byrappa, K.; Chandrashekar, B.N.; Ramakrishna, S.; et al. Novel green biomimetic approach for synthesis of ZnO-Ag nanocomposite; antimicrobial activity against food-borne pathogen, biocompatibility and solar photocatalysis. Sci. Rep. 2019, 9, 8303. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Kumar, M.; Curtis, A.; Hoskins, C. Application of nanoparticle technologies in the combat against anti-microbial resistance. Pharmaceutics 2018, 10, 11. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y. Biomedical potential of ultrafine Ag/AgCl nanoparticles coated on graphene with special reference to antimicrobial performances and burn wound healing. ACS Appl. Mater. Interfaces 2016, 8, 15067–15075. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, M.; Andronescu, E.; Dolete, G.; Popescu, R.C.; Fufă, O.; Chifiriuc, M.C.; Mogoantă, L.; Bălşeanu, T.A.; Mogoşanu, G.D.; Grumezescu, A.M.; et al. Silver nanocoatings for reducing the exogenous microbial colonization of wound dressings. Materials (Basel) 2016, 9, 345. [Google Scholar] [CrossRef]
- Naik, K.; Kowshik, M. The silver lining: Towards the responsible and limited usage of silver. J. Appl. Microbiol. 2017, 123, 1068–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, D.; Cura, F.; Candotto, V.; Gaudio, R.M.; Mucchi, D.; Carinci, F. Evaluation of the efficacy of titanium dioxide with monovalent silver ions covalently linked (TIAB) as an adjunct to scaling and root planning in the management of chronic periodontitis using PCR analysis: A microbiological study. J. Biol. Regul. Homeost. Agents 2015, 29, 127–130. [Google Scholar] [PubMed]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Dhom, J.; Bloes, D.A.; Peschel, A.; Hofmann, U.K. Bacterial adhesion to suture material in a contaminated wound model: Comparison of monofilament, braided, and barbed sutures. J. Orthop. Res. 2017, 35, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Ng, T.M.; Teng, C.; Ang, B.; Tai, H.Y.; Lye, D.C. Non concordance with surgical site infection prevention guidelines and rates of surgical site infections for general surgical, neurological, and orthopedic procedures. Antimicrob. Agents Chemother. 2011, 55, 4659–4663. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, A.; Schneider, J.; Wehner, S.; Matl, F.D.; Schieker, M.; von Eisenhart-Rothe, R.; Stemberger, A.; Burgkart, R. Novel high efficient coatings for anti-microbial surgical sutures using chlorhexidine in fatty acid slow-release carrier systems. PLoS ONE 2014, 9, e101426. [Google Scholar] [CrossRef] [PubMed]
- Baracs, J.; Huszár, O.; Sajjadi, S.G.; Horváth, O.P. Surgical site infections after abdominal closure in colorectal surgery using triclosan-coated absorbable suture (PDS Plus) vs. uncoated sutures (PDS II): A randomized multicenter study. Surg. Infect. (Larchmt) 2011, 12, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mingmalairak, C.; Ungbhakorn, P.; Paocharoen, V. Efficacy of antimicrobial coating suture coated polyglactin 910 with tricosan (Vicryl plus) compared with polyglactin 910 (Vicryl) in reduced surgical site infection of appendicitis, double blind randomized control trial, preliminary safety report. J. Med. Assoc. Thai. 2009, 92, 770–775. [Google Scholar] [PubMed]
- Edmiston, C.E.; Seabrook, G.R.; Goheen, M.P.; Krepel, C.J.; Johnson, C.P.; Lewis, B.D.; Brown, K.R.; Towne, J.B. Bacterial adherence to surgical sutures: Can antibacterial-coated sutures reduce the risk of microbial contamination? J. Am. Coll. Surg. 2006, 203, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Rothenburger, S.; Nichols, M.M. In vivo and in vitro antibacterial efficacy of PDS plus (polidioxanone with triclosan) suture. Surg. Infect. (Larchmt) 2008, 9, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Yazdankhah, S.P.; Scheie, A.A.; Høiby, E.A.; Lunestad, B.T.; Heir, E.; Fotland, T.Ø.; Naterstad, K.; Kruse, H. Triclosan and antimicrobial resistance in bacteria: An overview. Microb. Drug Resist. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.E.; Larson, E.L.; Levy, S.B. Consumer antibacterial soaps: Effective or just risky? Clin. Infect. Dis. 2007, 45, S137–S147. [Google Scholar] [CrossRef]
- Obermeier, A.; Schneider, J.; Harrasser, N.; Tübel, J.; Mühlhofer, H.; Pförringer, D.; Deimling, C.V.; Foehr, P.; Kiefel, B.; Krämer, C.; et al. Viable adhered Staphylococcus aureus highly reduced on novel antimicrobial sutures using chlorhexidine and octenidine to avoid surgical site infection (SSI). PLoS ONE 2018, 13, e0190912. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved standard M07-A9, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Volume 32. [Google Scholar]
- Pettit, R.K.; Weber, C.A.; Kean, M.J.; Hoffmann, H.; Pettit, G.R.; Tan, R.; Franks, K.S.; Horton, M.L. Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 2005, 49, 2612–2617. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Reinbold, J.; Uhde, A.K.; Müller, I.; Weindl, T.; Geis-Gerstorfer, J.; Schlensak, C.; Wendel, H.P.; Krajewski, S. Preventing surgical site infections using a natural, biodegradable, antibacterial coating on surgical sutures. Molecules 2017, 22, 1570. [Google Scholar] [CrossRef]
- Grande, R.; Di Marcantonio, M.C.; Robuffo, I.; Pompilio, A.; Celia, C.; Di Marzio, L.; Paolino, D.; Codagnone, M.; Muraro, R.; Stoodley, P.; et al. Helicobacter pylori ATCC 43629/NCTC 11639 Outer Membrane Vesicles (OMVs) from biofilm and planktonic phase associated with extracellular DNA (eDNA). Front. Microbiol. 2015, 6, 1369. [Google Scholar] [CrossRef]
Sample Availability: Samples of the MD and surgical sutures are available from the authors. |







| Bacterial Strains | MIC (mg/mL) | MBC (mg/mL) | MBIC (mg/mL) |
|---|---|---|---|
| S. aureus ATCC 29213 | 2 | 4 | 1.5 |
| E. coli ATCC 25922 | >8 | >8 | n.t. |
| E. faecalis ATCC 29212 | 2 | 4 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puca, V.; Traini, T.; Guarnieri, S.; Carradori, S.; Sisto, F.; Macchione, N.; Muraro, R.; Mincione, G.; Grande, R. The Antibiofilm Effect of a Medical Device Containing TIAB on Microorganisms Associated with Surgical Site Infection. Molecules 2019, 24, 2280. https://doi.org/10.3390/molecules24122280
Puca V, Traini T, Guarnieri S, Carradori S, Sisto F, Macchione N, Muraro R, Mincione G, Grande R. The Antibiofilm Effect of a Medical Device Containing TIAB on Microorganisms Associated with Surgical Site Infection. Molecules. 2019; 24(12):2280. https://doi.org/10.3390/molecules24122280
Chicago/Turabian StylePuca, Valentina, Tonino Traini, Simone Guarnieri, Simone Carradori, Francesca Sisto, Nicola Macchione, Raffaella Muraro, Gabriella Mincione, and Rossella Grande. 2019. "The Antibiofilm Effect of a Medical Device Containing TIAB on Microorganisms Associated with Surgical Site Infection" Molecules 24, no. 12: 2280. https://doi.org/10.3390/molecules24122280
APA StylePuca, V., Traini, T., Guarnieri, S., Carradori, S., Sisto, F., Macchione, N., Muraro, R., Mincione, G., & Grande, R. (2019). The Antibiofilm Effect of a Medical Device Containing TIAB on Microorganisms Associated with Surgical Site Infection. Molecules, 24(12), 2280. https://doi.org/10.3390/molecules24122280