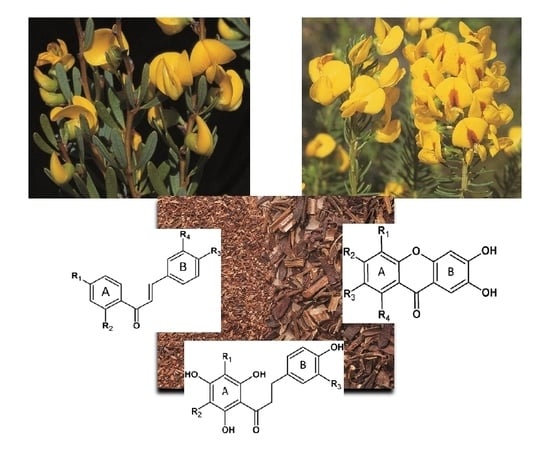
Patterns of Variation and Chemosystematic Significance of Phenolic Compounds in the Genus Cyclopia (Fabaceae, Podalyrieae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Tentative Identification of New Compounds in Cyclopia in Table 1
2.2. Levels of Main Compounds
2.3. Old Samples Versus Contemporary Samples
2.4. Differences Between Plant Parts (Twigs, Leaves, Pods, Flowers And Seeds)
2.5. Diagnostic Value of Phenolic Compounds
3. Conclusions
4. Materials and Methods
4.1. Samples and Sampling
4.2. Extraction
4.3. Standards
4.4. UPLC-HRMS Analysis
4.5. Data Processing and Clustering
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Wyk, B.-E.; Gorelik, B. The history and ethnobotany of Cape herbal teas. S. Afr. J. Bot. 2017, 110, 18–38. [Google Scholar] [CrossRef]
- Joubert, E.; Gelderblom, W.C.A.; Louw, A.; De Beer, D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides–A review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Joubert, M.E.; Bester, C.; De Beer, D.; De Lange, J.H. Honeybush (Cyclopia spp.): From local cottage industry to global markets- the catalytic and supporting role of research. S. Afr. J. Bot. 2011, 77, 887–907. [Google Scholar] [CrossRef]
- Hofmeyr, E.P.; Phillips, P. The genus Cyclopia Vent. Bothalia 1922, 1, 105–109. [Google Scholar] [CrossRef]
- Kies, P. Revision of the Genus Cyclopia and Notes on Some Other Sources of Bush Tea. Bothalia 1951, 6, 161–176. [Google Scholar] [CrossRef]
- Schutte, A.L. A taxonomic study of the tribes Podalyrieae and Liparieae (Fabaceae). Ph.D. Thesis, University of Johannesburg, Auckland Park, South Africa, 1995. [Google Scholar]
- Schutte, A.L. Systematics of the genus Cyclopia Vent. (Fabaceae, Podalyrieae). Edinb. J. Bot. 1997, 54, 125–170. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Gericke, N. People’s plants: A Guide to Useful Plants of Southern Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2018. [Google Scholar]
- Van Wyk, B.-E. The value of chemosystematics in clarifying relationships in the genistoid tribes of papilionoid legumes. Biochem. Syst. Ecol. 2003, 31, 875–884. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Schutte, A.L. Phylogenetic relationships in the tribes Podalyrieae, Liparieae and Crotalarieae. Adv. Legume Syst. 1995, 7, 283–308. [Google Scholar]
- Schutte, A.L.; Van Wyk, B.-E. Evolutionary relationships in the Podalyrieae and Liparieae based on morphological, cytological and chemical evidence. Plant Syst. Evol. 1998, 209, 1–31. [Google Scholar] [CrossRef]
- Van Der Bank, M.; Van Wyk, B.-E.; Van Der Bank, H. Biochemical Genetic Variation in Four Wild Populations of Aspalathus linearis (Rooibos Tea). Biochem. Syst. Ecol. 1995, 23, 257–262. [Google Scholar] [CrossRef]
- Van der Bank, M.; Chase, M.W.; Van Wyk, B.-E.; Fay, M.F.; Van der Bank, H.; Reeves, G.; Hulme, A. Systematics of the tribe Podalyrieae (Fabaceae) based on DNA, morphological and chemical data. Bot. J. Linn. Soc. 2002, 139, 159–170. [Google Scholar] [CrossRef]
- De Nysschen, A.M.; Van Wyk, B.-E.; Van Heerden, F.R. The major phenolic compounds in the leaves of Cyclopia species (honeybush tea). Biochem. Syst. Ecol. 1996, 24, 243–246. [Google Scholar] [CrossRef]
- De Nysschen, A.M.; Van Wyk, B.-E.; Van Heerden, F. Seed flavonoids of the tribes Podalyrieae and Liparieae (Fabaceae). Plant Syst. Evol. 1998, 212, 1–11. [Google Scholar] [CrossRef]
- Kamara, B.I.; Brand, D.J.; Brandt, E.V.; Joubert, E. Phenolic metabolites from Honeybush tea (Cyclopia subternata). J. Agric. Food Chem. 2004, 52, 5391–5395. [Google Scholar] [CrossRef]
- Kokotkiewicz, M.; Luczkiewicz, A. Review: Honeybush (Cyclopia sp.)-A rich source of compounds with high antimutagenic properties. Fitoterapia 2009, 80, 3–11. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Schulze, A.E.; Joubert, E.; De Villiers, A.; Malherbe, C.J.; Stander, M.A. Food ingredient extracts of Cyclopia subternata (honeybush): Variation in phenolic composition and antioxidant capacity. Molecules 2012, 17, 14606–14624. [Google Scholar] [CrossRef]
- Beelders, T.; De Beer, D.; Stander, M.A.; Joubert, E. Comprehensive phenolic profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and -MS/MS reveals novel xanthone and benzophenone constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef]
- Roza, O.; Martins, A.; Hohmann, J.; Lai, W.; Eloff, J.; Chang, F.; Csupor, D. Flavonoids from Cyclopia genistoides and their Xanthine Oxidase Inhibitory Activity. Planta Med. 2016, 82, 1274–1278. [Google Scholar] [CrossRef]
- Walters, N.A.; De Beer, D.; De Villiers, A.; Walczak, B.; Joubert, E. Genotypic variation in phenolic composition of Cyclopia pubescens (honeybush tea) seedling plants. J. Food Compos. Anal. 2019, 78, 129–137. [Google Scholar] [CrossRef]
- Singh, S.P.; Ali, M.M.; Jain, G.K. High-throughput Quantification of Isoflavones, Biochanin A and Genistein, and their Conjugates in Female Rat Plasma using LC-ESI-MS/MS: Application in Pharmacokinetic Study. J. Sep. Sci. 2010, 33, 3326–3334. [Google Scholar] [CrossRef]
- Yang, M.; Ye, W.; Meng, G.; Sabir, A.; Qiao, G.; Guo, X.; Ye, D. Rapid Characterisation of Flavonoids from Sophora alopecuroides L. by HPLC/DAD/ESI-MSn. Nat. Prod. Res. 2013, 27, 232–330. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.V.; Kotina, E.L.; Tilney, P.M.; Van Wyk, B.-E. Leaf and stem anatomy of honeybush tea (Cyclopia species, Fabaceae). S. Afr. J. Bot. 2012, 82, 123–128. [Google Scholar] [CrossRef]
- Stander, M.A.; Brendler, T.; Redelinghuys, H.; Van Wyk, B.-E. The commercial history of Cape herbal teas and an analysis of phenolic compounds in historic teas from a depository of 1933. J. Food Compos. Anal. 2019, 76, 66–73. [Google Scholar] [CrossRef]
- Ferreira, D.; Kamara, B.I.; Brandt, E.V.; Joubert, E. Phenolic compounds from Cyclopia intermedia (Honeybush Tea). J. Agric. Food Chem. 1998, 46, 3406–3410. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |














| Retention Time | Exprimental m/z | Formula | MSE Fragments | Reference | ||
|---|---|---|---|---|---|---|
| 1 | 3.64 | 191.0197 | C6H7O7 | 191.0197,111.0087,87.0082,85.0303 | *Citric acid | New |
| 2 | 5.18 | 325.1131 | C12H21O10 | 325.1143,193.0726,161.0428,101.0237 | Ferulic acid pentoside (arabinose) isomer1 | New |
| 3 | 5.73 | 325.1127 | C12H21O10 | 325.1143,193.0712,161.0491,101.0237 | Ferulic acid pentoside isomer2 | New |
| 4 | 6.06 | 339.1286 | C13H23O10 | 339.1292,193.0725,161.0461,101.0260 | Ferulic acid rhamnose isomer1 | New |
| 5 | 6.72 | 339.1286 | C13H23O10 | 339.1268,207.0880,178.8859,161.0460,113.0221,101.0234 | Ferulic acid rhamnose isomer2 | New |
| 6 | 8.82 | 255.0509 | C11H11O7 | 255.0509,165.0547,72.9930 | Piscidic acid | New |
| 7 | 9.13 | 569.1503 | C25H29O15 | 569.1558,449.1093,287.0552,167.0341,125.0242 | Iriflophenone-di-O,C-hexoside (3-β-D-glucopyranosyl-4-β-D-glucopyranosyloxyiriflophenone) | [18,19,20,21] |
| 8 | 8.98 | 153.0189 | C7H5O4 | 153.0192,109.0305 | *Protocatechuic acid (dihydroxybenzoic acid) | New |
| 9 | 9.79 | 431.1552 | C19H27O11 | 431.1524,293.0834,233.0672,89.0247 | Unknown 431_9.8 | New |
| 10 | 9.87 | 423.0922 | C19H19O11 | 423.0932,303.0525,193.0142,109.0294 | Maclurin-3-C-glucoside (3-β-D-glucopyranosylmaclurin) | [18,19,20,21] |
| 11 | 10.77 | 285.0621 | C12H13O8 | 285.0622, 153.0184,152.0117,109.0285,108.0222 | Dihydroxybenzoic acid-O-pentoside | [19] |
| 12 | 11.8 | 583.1301 | C25H27O16 | 583.1302,421.0778,331.0447,301.0369,272.0332,259.0255 | (Iso)Mangiferin-O-hexoside (tetrahydroxyxanthone-di-O,C-hexose) | [21] |
| 13 | 11.21 | 429.1401 | C19H25O11 | 429.1383,135.0452 | Piceol-hexoside-pentoside isomer1 | New |
| 14 | 11.39 | 429.1400 | C19H25O11 | 429.1404,293.0877,233.0666,135.0456 | Piceol-hexoside-pentoside isomer2 | New |
| 15 | 11.58 | 431.1555 | C19H27O11 | 431.1552,275.0564,163.0406,119.0432 | Unknown 431_11.6 | New |
| 16 | 11.68 | 443.1558 | C20H27O11 | 135.0453,96.9698 | Piceol-hexoside-rhamnoside (Sibiricaphenone) | New |
| 17 | 12.04 | 407.0981 | C19H19O10 | 407.0979,317.0664,287.0555,245.0453,193.0129,125.0247 | Iriflophenone-3-C-glucoside (3-β-D-glucopyranosyliriflophenone) | [18,19,20,21] |
| 18 | 12.15 | 417.1046 | C17H21O12 | 417.1038,153.0178,152.0110,109.0285,108.0222 | Dihydroxybenzoic acid-O-dipentoside | [19] |
| 19 | 12.47 | 325.0918 | C15H17O8 | 325.0942,163.0406,119.0503 | p-Coumaric acid hexoside | New |
| 20 | 12.8 | 457.1352 | C20H25O12 | 457.1357,163.0401,119.0498 | p-coumaric acid-O-pentose-O-hexoside1 | [19] |
| 21 | 13.15 | 457.1351 | C20H25O12 | 457.1342,163.0405,145.0300,119.0494 | p-coumaric acid-O-pentose-O-hexoside2 | [19] |
| 22 | 13.27 | 457.1352 | C20H25O12 | 457.1342,163.0403,119.0496 | p-coumaric acid-O-pentose-O-hexoside3 | [19] |
| 23 | 13.69 | 401.1446 | C18H25O10 | 401.1446,269.1029,179.0345,161.0448,101.0240 | Unknown 401_13.6 | New |
| 24 | 13.92 | 595.1644 | C27H31O15 | 595.1658,459.1141,433.1251,287.0541,169.0142,161.0269,151.0044,135.0444,125.0245 | Eriodictyol-O-hexose-O-rhamnose isomer1 | [18,19,21] |
| 25 | 14.06 | 457.1709 | C21H29O11 | 457.1703,293.0873,233.0671,149.0464,125.0249,89.0246 | Unknown 457_14 | New |
| 26 | 14.3 | 421.0764 | C19H17O11 | 421.0768,301.0358,331.0441,259.0246 | *Mangiferin | [14,17,18,19,21] |
| 27 | 14.48 | 595.1651 | C27H31O15 | 595.1658,459.1141,287.0541,169.0142,161.0269,151.0044,135.0444,125.0245 | Eriodictyol-O-hexose-O-rhamnose isomer2 | [18,19,21] |
| 28 | 14.67 | 421.0763 | C19H17O11 | 421.0771,301.0347,331.0458,258.0170 | Isomangiferin | [18,19,21] |
| 29 | 14.91 | 381.1767 | C16H29O10 | 381.1767,249.1344,161.0453,101.0256,96.9703 | Unknown 381_14.9 | New |
| 30 | 15.15 | 465.1031 | C21H21O12 | 465.1046,285.0407,151.0042 | Unknown 465_15.15 | New |
| 31 | 14.33 | 449.1079 | C21H21O11 | 449.1081,287.0551,269.0448,259.0616,163.0038,135.0086,121.0290,109.0296 | Eriodictyol-O-glucoside isomer1 | [17] |
| 32 | 15.69 | 449.1079 | C21H21O11 | 449.1079,287.0553, 269.0450,259.0616,225.0561,151.0035,135.0448 | Eriodictyol-O-glucoside isomer2 | [18] |
| 33 | 15.87 | 579.1725 | C27H31O14 | 579.1765,271.0618,151.0027,145.0300,125.0260,119.0489 | Naringenin-O-hexoside-O-rhamnose isomer1 | [19] |
| 34 | 16.11 | 415.1621 | C19H27O10 | 415.1585,273.0681,149.0466,137.0246,101.0249,89.0247 | Unknown 415_16.1 | New |
| 35 | 16.31 | 579.1701 | C27H31O14 | 579.1689,271.0633,151.0022,145.0282,125.0253,119.0500 | Naringenin-O-hexoside-O-rhamnose isomer2 | [19] |
| 36 | 16.47 | 447.093 | C21H19O11 | 447.0956,285.0415,284.0320,255.0299,119.0452,96.9697 | Orobol/Luteolin-O-hexoside1 | New |
| 37 | 16.63 | 613.1776 | C27H33O16 | 613.1766,505.1346,493.1363,433.1129,403.1020,373.0938,331.0838,251.0536,209.0461 | 3-hydroxyphloretin-3′,5′-di-C-hexoside | [19] |
| 38 | 16.99 | 463.2177 | C21H35O11 | 463.2181,251.0763,191.0575,149.0461,96.9692,89.0249 | Unknown 463_17 | New |
| 39 | 17.37 | 463.2186 | C21H35O11 | 463.2188,251.0777,191.0567,149.0456,96.9700,89.0250 | Unknown 463_17.4 | New |
| 40 | 17.55 | 595.1657 | C27H31O15 | 595.1658,459.1141,433.1251,287.0541,169.0142,161.0269,151.0044,135.0444,125.0245 | Eriodictyol-O-hexose-O-rhamnose isomer3 | [19] |
| 41 | 17.59 | 433.1133 | C21H21O10 | 433.1153,271.0600,151.0022 | Naringenin-O-hexoside isomer1 | New |
| 42 | 17.88 | 433.1133 | C21H21O10 | 433.1153,271.0600,151.0022 | Naringenin-O-hexoside isomer2 | New |
| 43 | 18.18 | 593.1505 | C27H29O15 | 593.1522,285.0408 | *Luteolin-O-rutinoside (Scolymoside) | [18,19] |
| 44 | 18.28 | 487.1812 | C22H31O12 | 487.1812,191.0563,149.0456,101.0245,89.0247 | Unknown 487_18.3 | New |
| 45 | 18.47 | 597.1815 | C27H33O15 | 597.1801,477.1390,417.1172,387.1068,357.0969,209.0449,167.0363, 125.0236 | Phloretin-3′,5′-di-C-glucoside | [19] |
| 46 | 18.59 | 433.1129 | C21H21O10 | 433.1133,271.0607,135.0452,91.0191 | Naringenin-O-hexoside isomer3 | New |
| 47 | 18.73 | 447.0942 | C21H19O11 | 447.0956,285.0414,284.0334 | Orobol/Kaempferol/Luteolin-O-hexoside2 | New |
| 48 | 19.34 | 595.1661 | C27H31O15 | 595.1654,459.1166,287.0532,161.0247,151.0033,135.0462,125.0247 | Eriodictyol-O-hexose-O-rhamnose isomer4 | [19] |
| 49 | 19.53 | 579.1732 | C27H31O14 | 579.1657,271.0623,151.0035,145.0300,125.0260,119.0486,96.9697 | Naringenin-O-hexoside-O-rhamnose isomer3/Narirutin | [21] |
| 50 | 19.83 | 417.1176 | C21H21O9 | 417.1171,211.0763,169.0662,98.0241 | Unknown 417_19.8 isomer1 | New |
| 51 | 20.17 | 445.1141 | C22H21O10 | 445.1138,283.0615,268.0378,239.0379 | Olmelin-O-hexoside | New |
| 52 | 20.77 | 609.1811 | C28H33O15 | 609.1781,301.0717,286.0483 | *Hesperidin (Hesperetin-O-rutinoside) | [18] |
| 53 | 20.74 | 579.1681 | C27H31O14 | 579.1765,271.0617,151.0031,145.0292,125.0250,119.0492,96.9690 | Naringenin-O-hexoside-O-rhamnose isomer4 | [19] |
| 54 | 20.99 | 527.1194 | C26H23O12 | 527.1194, 317.0669, 287.0562,245.0457,193.0141 | Unknown 527_20.99 | New |
| 55 | 21 | 593.2447 | C26H41O15 | 547.2388,515.2121,96.9693 | Unknown 593_21 | New |
| 56 | 21.16 | 271.0612 | C15H11O5 | 271.0612,135.0449,96.9697, 91.0187 | Butein/Butin | [15] |
| 57 | 21.3 | 549.1619 | C26H29O13 | 549.1622,301.0710,255.0663,237.0594,211.0773,125.0275,89.0239 | Unknown 549_21.3 | New |
| 58 | 21.67 | 593.1506 | C27H29O15 | 593.1525,457.1313,417.1021,399.0924,287.0583,163.0395,152.0112,119.0485,96.9688 | Unknown 593_21.6 | New |
| 59 | 22.34 | 433.113 | C21H21O10 | 433.1129,271.0602,135.0448,91.0189 | Butein-hexoside isomer1 | New |
| 60 | 22.3 | 417.1193 | C21H21O9 | 417.1171,211.0763,169.0662,98.0241 | Unknown 417_22.3 isomer2 | New |
| 61 | 23.49 | 433.1147 | C21H21O10 | 433.1145,271.0619,135.0456,91.0194 | Butein-hexoside isomer2 | New |
| 62 | 24.39 | 285.0359 | C15H9O6 | 285.0404,161.0290,151.0016,135.0422 | Orobol | [15,16] |
| 63 | 24.4 | 287.0561 | C15H11O6 | 287.0561,151.0038,135.0452 | Eriodictyol | [26] |
| 64 | 24.7 | 593.1856 | C28H33O14 | 593.1882,285.0759,243.0666,151.0045 | Didymin/Neoponcirin (Isosakuranetin-7-O-rutinoside) | New |
| 65 | 24.79 | 447.2226 | C21H35O10 | 447.2246,315.1848,161.0459,101.0243,96.9688,113.0239,71.0130 | Unknown 447_25 | New |
| 66 | 25.03 | 285.0402 | C15H9O6 | 285.0404,175.0396,151.0051,133.0301 | *Luteolin | [25] |
| 67 | 25.35 | 285.0783 | C16H13O5 | 285.0400,255.0698,163.0379,135.0315 | Unknown 285_25.35 | New |
| 68 | 25.93 | 301.2021 | C16H29O5 | 301.2024,96.9695 | Unknown 301_25.9 | New |
| 69 | 26.36 | 271.0609 | C15H11O5 | 271.0620,151.0036,119.0500,107.0136,96.9683 | *Naringenin | [26] |
| 70 | 27.05 | 327.217 | C18H31O5 | 327.2184,229.1416,211.1331,171.1022 | Unknown 327_27 | New |
| 71 | 27.23 | 301.0713 | C16H13O6 | 301.0712,286.0497,164.0111,151.0034,136.0181 | *Hesperetin | [15,26] |
| 72 | 28.29 | 287.2221 | C16H31O4 | 287.2211,96.9678,78.9490 | Unknown 287_28.3 (hydroxylated fatty acid?) | New |
| 73 | 28.92 | 285.2067 | C16H29O4 | 285.2070,96.9668 | Unknown 285_28.92 | New |
| 74 | 30.15 | 285.0763 | C16H13O5 | 285.0760,270.0516,243.0666,164.0114,151.0030,136.0164,108.0216 | (Iso)sakuranetin | [14] |
| Sample Number | Species | Sample Code | Provenance | VOUCHER SPECIMEN | Date | Part(s) Analysed |
|---|---|---|---|---|---|---|
| Collected | ||||||
| 1 | Cyclopia aurescens Kies | AU1L | Klein Swartberg | Schutte & Van Wyk 771a | 3/2/1992 | leaves |
| 2 | AU2T | Klein Swartberg | Schutte & Van Wyk 771a | 3/2/1992 | twigs | |
| 3 | AU3P | Klein Swartberg | Schutte & Van Wyk 771a | 3/2/1992 | pods | |
| 4 | AU4S | Klein Swartberg | Schutte & Van Wyk 771a | 3/2/1992 | seeds | |
| 5 | AU5P | Klein Swartberg | Schutte & Van Wyk 775 | 3/2/1992 | pods | |
| 6 | AU5S | Klein Swartberg | Schutte & Van Wyk 775 | 3/2/1992 | seeds | |
| 7 | Cyclopia bolusii Hofmeyr & E.Phillips | BO1L | Swartberg Pass | Schutte & Vlok 749 | 1/2/1992 | leaves |
| 8 | BO2T | Swartberg Pass | Schutte & Vlok 749 | 1/2/1992 | twigs | |
| 9 | BO3P | Swartberg Pass | Schutte & Vlok 749 | 1/2/1992 | pods | |
| 10 | Cyclopia bowieana Harv. | BW1L | Ruitersberg | Schutte 526 | 1/1990 | leaves |
| 11 | BW2T | Ruitersberg | Schutte 526 | 1/1990 | twigs | |
| 12 | BW3P | Ruitersberg | Schutte 526 | 1/1990 | pods | |
| 13 | BW4S | Ruitersberg | Schutte 526 | 1/1990 | seeds | |
| 14 | Cyclopia burtonii Hofmeyr & E.Phillips | BU1L | Swartberg | Schutte 641 | 9/1990 | leaves |
| 15 | BU2T | Swartberg | Schutte 641 | 9/1990 | twigs | |
| 16 | BU3L | Swartberg Pass | Schutte 643 | 9/1990 | leaves | |
| 17 | BU4T | Swartberg Pass | Schutte 643 | 9/1990 | twigs | |
| 18 | BU5L | Swartberg Pass | Schutte 747 | 1/2/1992 | leaves | |
| 19 | BU6T | Swartberg Pass | Schutte 747 | 1/2/1992 | twigs | |
| 20 | Cyclopia buxifolia (Burm.f.) Kies | BX1L | Jonkershoek | Schutte 604 | 9/1990 | leaves |
| 21 | BX2T | Jonkershoek | Schutte 604 | 9/1990 | twigs | |
| 22 | BX3L | Jonkershoek | Schutte 605 | 9/1990 | leaves | |
| 23 | BX4L | Jonkershoek | Schutte 606 | 9/1990 | leaves | |
| 24 | Cyclopia capensis T.M.Salter | CA1L | Cape Point | Schutte 550 | 1/1990 | Leaves |
| 25 | CA2T | Cape Point | Schutte 550 | 1/1990 | twigs | |
| 26 | Cyclopia falcata (Harv.) Kies | FA1L | Franschoek Pass | Schutte 612 | 9/1990 | leaves |
| 27 | FA2T | Franschhoek Pass | Schutte 612 | 9/1990 | twigs | |
| 28 | Cyclopia genistoides (L.) R.Br. | GE1L | Constantia Mountain | Schutte 615 | 14/09/1990 | leaves |
| 29 | GE2L | Constantia Mountain | Van Wyk 2747 | 16/1/1988 | leaves | |
| 30 | GE3L | Rooiels | Schutte 622 | 15/9/1990 | leaves | |
| 31 | GE4L | Bettys Bay | Schutte 624 | 15/9/1990 | leaves | |
| 32 | GE5L | Bettys Bay | Schutte 624 | 15/9/1990 | leaves | |
| 33 | GE6T | Bettys Bay | Schutte 624 | 15/9/1990 | twigs | |
| 34 | GE7L | Bettys Bay | Schutte 624 | 15/9/1990 | leaves | |
| 35 | GE8L | Bettys Bay | Schutte 625 | 15/9/1990 | leaves | |
| 36 | GE9L | Buffelshoek, Albertinia | Vlok 2249 | 28/11/1989 | leaves | |
| 37 | GE10L | De Hoop | Boatwright & Magee 53 | 25/11/2004 | leaves | |
| 38 | Cyclopia glabra (Hofmeyr & E.Phillips) A.L. Schutte | GL1L | Matroosberg | Schutte 557 | 01/2/1990 | leaves |
| 39 | GL2F | Matroosberg | Schutte 557 | 01/2/1990 | flowers | |
| 40 | IN1L | Anysberg | Schutte 680 | 9/1990 | leaves | |
| 41 | Cyclopia intermedia E.Mey. | IN2T | Anysberg | Schutte 680 | 9/1990 | twigs |
| 42 | IN3L | Touwsberg | Van Wyk, Winter & Tilney 3416 | 05/10/1993 | leaves | |
| 43 | IN4L | Oudtshoorn | Schutte 521 | 24/1/1990 | leaves | |
| 44 | IN5L | Teeberg | Schutte 524 | 25/1/1990 | leaves | |
| 45 | IN6L | Teeberg | Schutte 724b & c | 1/1992 | leaves | |
| 46 | IN7L | Swartberg Pass | Schutte 646 | 17/9/1990 | leaves | |
| 47 | IN8L | Swartberg Pass | Schutte 647 | 17/9/1990 | leaves | |
| 48 | IN9L | Prince Alfred’s Pass | Van Wyk 928 | 20/2/1982 | leaves | |
| 49 | IN10L | Prince Alfred’s Pass | Schutte 578 | 23/1/1990 | leaves | |
| 50 | IN11L | K’Buku, De Vlug | Van Wyk 945 | 20/2/1982 | leaves | |
| 51 | IN12L | K’Buku, De Vlug | Van Wyk 947 | 20/2/1982 | leaves | |
| 52 | IN13L | K’Buku, De Vlug | Van Wyk 951 | 20/2/1982 | leaves | |
| 53 | IN14L | Joubertina | Schutte 507 | 22/01/1990 | leaves | |
| 54 | IN15L | Hoopsberg | Schutte 513 | 23/01/1990 | leaves | |
| 55 | IN16L | Hoopsberg | Schutte 573 | 1/1990 | leaves | |
| 56 | IN17L | Hoopsberg | Schutte 573 | 1/1990 | twigs | |
| 57 | Cyclopia maculata (Andrews) Kies | MA1L | Garcia State Forest | Schutte 528b | 26/01/1990 | leaves |
| 58 | MA2L | Garcia State Forest | Van Wyk 895 | 02/10/1981 | leaves | |
| 59 | MA3L | Garcia State Forest | Schutte 528 | 1/1990 | leaves | |
| 60 | MA4T | Garcia State Forest | Schutte 528 | 1/1990 | twigs | |
| 61 | Cyclopia meyeriana Walp. | ME1L | Matroosberg | Schutte 557 | 1/2/1990 | leaves |
| 62 | ME2T | Matroosberg | Schutte 557 | 1/2/1990 | twigs | |
| 63 | Cyclopia plicata Kies | PL1L | Hoopsberg | Schutte 670a | 09/1990 | leaves |
| 64 | PL2T | Hoopsberg | Schutte 670a | 09/1990 | twigs | |
| 65 | PL3L | Hoopsberg | Schutte 670b | 09/1990 | leaves | |
| 66 | PL4T | Hoopsberg | Schutte 670b | 09/1990 | twigs | |
| 67 | Cyclopia pubescens Eckl. & Zeyh. | PU1L | Port Elizabeth | Schutte 685 | 22/9/1990 | leaves |
| 68 | PU2T | Port Elizabeth | Schutte 685 | 22/9/1990 | twigs | |
| 69 | PU3L | Port Elizabeth | Schutte 686 | 22/9/1990 | leaves | |
| 70 | PU4L | Port Elizabeth | Schutte 687 | 22/9/1990 | leaves | |
| 71 | PU5L | Port Elizabeth | Schutte 688 | 22/9/1990 | leaves | |
| 72 | Cyclopia subternata Vogel | SU1L | Bloukrantz River | Schutte 683 | 21/09/1990 | leaves |
| 73 | SU2L | Bloukrantz River | Schutte 683 | 9/1990 | leaves | |
| 74 | SU3L | Kareedouw Pass | Schutte 505 | 22/01/1990 | leaves | |
| 75 | SU4L | Prince Alfred’s Pass | Schutte 519 | 23/01/1990 | leaves | |
| 76 | SU5L | Prince Alfred’s Pass | Van Wyk 939 | 20/2/1985 | leaves | |
| 77 | SU6L | Outeniqua Pass | Schutte 639 | 9/1990 | leaves | |
| 78 | SU7T | Outeniqua Pass | Schutte 639 | 9/1990 | twigs | |
| 79 | SU8L | Outeniqua Pass | Schutte 690b | 08/09/1991 | leaves | |
| 80 | SU9F | Outeniqua Pass | Schutte 690b | 08/09/1991 | flowers | |
| 81 | SU10L | Witelsbos | Schutte 503 | 22/01/1990 | leaves | |
| 82 | SU11L | Elandsbos River | Schutte s.n. 1b | 9/1990 | leaves |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stander, M.A.; Redelinghuys, H.; Masike, K.; Long, H.; Van Wyk, B.-E. Patterns of Variation and Chemosystematic Significance of Phenolic Compounds in the Genus Cyclopia (Fabaceae, Podalyrieae). Molecules 2019, 24, 2352. https://doi.org/10.3390/molecules24132352
Stander MA, Redelinghuys H, Masike K, Long H, Van Wyk B-E. Patterns of Variation and Chemosystematic Significance of Phenolic Compounds in the Genus Cyclopia (Fabaceae, Podalyrieae). Molecules. 2019; 24(13):2352. https://doi.org/10.3390/molecules24132352
Chicago/Turabian StyleStander, Maria. A., Herman Redelinghuys, Keabetswe Masike, Helen Long, and Ben-Erik Van Wyk. 2019. "Patterns of Variation and Chemosystematic Significance of Phenolic Compounds in the Genus Cyclopia (Fabaceae, Podalyrieae)" Molecules 24, no. 13: 2352. https://doi.org/10.3390/molecules24132352
APA StyleStander, M. A., Redelinghuys, H., Masike, K., Long, H., & Van Wyk, B. -E. (2019). Patterns of Variation and Chemosystematic Significance of Phenolic Compounds in the Genus Cyclopia (Fabaceae, Podalyrieae). Molecules, 24(13), 2352. https://doi.org/10.3390/molecules24132352