1. Introduction
With the changes in human living habits, periodontal disease has become the "top chronic killer" in oral diseases [
1]. Periodontal treatment includes surgical and non-surgical methods. Underarm treatment and root smoothing can provide more recovery of the operation area, which, however, may also cause the additional risks of bleeding, pain, and infection in the patients’ gums during the treatments, increasing patient discomfort [
2]. In recent years, non-surgical methods have attracted more and more attention, with an aim to avoid the above problems in controlling and treating periodontal disease. Photodynamic therapy is one of the most important non-surgical treatment methods, combining special photosensitizers with bio-optical techniques. This phototherapy method in dentistry, also known as antimicrobial PDT (aPDT), displays efficient bactericidal performance for oral pathogens [
3]. With the emergence of drug-resistant strains due to the abuse of antibiotics and bacterial mutations, aPDT becomes especially important. The basic principles of photodynamic therapy are as follows: After the applied photosensitizer is attached to the bacteria, the laser light is introduced and the photosensitizer absorbs light energy into the singlet state from the stable triplet state. The singlet photosensitizer is extremely unstable and will instantaneously release the energy, returning to a triplet state [
4]. The released energy is absorbed by tissue oxygen, forming reactive oxygen species (ROS) which have strong oxidation and high reactivity, causing the rapid lipid oxidation of bacteria, especially the destruction of vulnerable membrane lipids, and eventually bacterial death [
5].
Antimicrobial PDT has been considered to be an important method in killing inflammation-related pathogens around teeth. Hass et al. firstly conducted the in vitro study on this point [
6]. Three bacteria,
Prevotella intermedia (
P. intermedia),
Aggregatibacter actimycetemcomitans (
A. actimycetemcomitans),
Porphyromonas gingivalis (
P. gingivalis), were selected and treated with aPDT method, respectively. All above three species were completely killed, confirming the high efficacy of bacteria inhibition compared with other treatment methods [
6]. Chan et al. used methylene blue (MB) as a photosensitizer to kill three kinds of bacteria,
P. gingivalis, A.
actimycetemcomitans and
Fusobacterium nucleatum (
F. nucleatum) with inhibition rate of 95% [
7]. Ayano
et al. investigated the aPDT effect on
P. gingivalis with photosensitizer rose bengal (RB) and the results show that aPDT from RB can effectively kill
P. gingivalis with blue light [
8]. Goulart et al. found
A. actimycetemcomitans can be effectively inhibited with MB under the irradiation of light from 400 to 500 nm, and the number of surviving bacteria was significantly reduced [
9]. Eick et al. investigated the aPDT from the toluidine blue (TB) with light excitation (625–635 nm), which can significantly reduce
A. actimycetemcomitans biofilm activity formed by two different strains [
10]. Street et al. combined the MB and lasers (650 to 675 nm) and found more effective treatment effect on
F. nucleatum planktonic bacteria than that of biofilms, indicating that the effect of PDT on bacteria is also related to the bacteria form [
11].
Although photodynamic therapy has made important progress in the treatment of periodontal disease, it is still in its primary stage, and there are some serious problems that are still necessary to be solved before clinical application. The most important one of conventional photodynamic therapy is the weak tissue penetration of ultraviolet or visible light. Therefore, it is highly desirable to design and prepare a photodynamic therapy system with the infrared irradiation light, which can penetrate deep tissues.
Upconversion fluorescence is an anti-stokes process that allows the long excitation wavelength to be converted to short emission wavelength. Rare earth doped upconversion nanoparticles (UCNPs) are the most outstanding representatives which can convert the infrared light to visible emission light by the continuous two-photon or multiphoton energy transfer process [
12,
13,
14]. Upconversion brings many advantages: First, there is zero noise in biological background because biological tissue does not emit light under near-infrared (NIR) light, resulting in high signal-to-noise ratio in applications [
15]. Second, the NIR excitation light used for up-conversion luminescence is located in the optical imaging window of the biological tissue, and has a deep tissue penetration. Red light can penetrate inside the tissue for 0.5 cm, while 980 nm light can penetrate more than 1 cm [
16]. Third, some other advantages should be mentioned, such as narrow emission band, high color purity and stability, low toxicity and no photobleaching [
17,
18]. Therefore, if the up-conversion material were introduced in the periodontal deep tissue, the NIR light can be converted into light in different wavelengths from ultraviolet to NIR, satisfying the need to excite photosensitizers with different absorption bands, and well compensating for the low penetration of conventional photodynamic therapy light sources.
Note that the PDT triggered by upconversion light was first designed and applied in tumor therapy [
19]. However, until now there have been no reports about such upconversion nanomaterials-based photodynamic therapy on periodontitis treatment, which is highly expected to be achieved if non-invasive periodontitis treatment is considered.
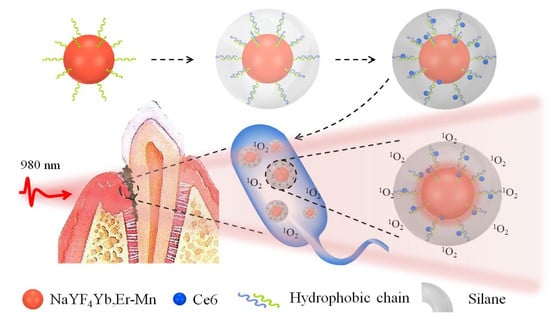
In this work, we designed a new UCNP/Ce6 composite nanomaterial with enhanced red light emission and efficient aPDT bactericidal performance. The combination of Ce6 and NaYF
4:Yb,Er UCNPs was realized by using the amphiphilic silane modification technique, which involved the hydrophobic-hydrophobic interaction between hydrophobic side chain of the silane and hydrophobic groups on the surface of UCNPs [
20]. In the NaYF
4:Yb,Er/Ce6 composites, after hydrolysis, the silane forms a very thin hydrophilic coating, which means the hydrophobic UCNPs were converted to hydrophilic ones without increasing size and influencing the luminescent centers on the surface of UCNPs, since the hydrophobic side chain of the silane does not touch the surface of UCNPs. In addition, this design successfully avoids the leakage problem in conventional photosensitizer carriers by physical adsorption, such as photosensitizer loading in mesopores UCNP@mSiO
2 NPs [
19], which may lead to serious leakage problems, causing systemic toxic side effects as well. As the PDT function of Ce6 molecule should be triggered by excitation of red light, Mn doping is involved in this work, which greatly improves the probability of the red emission transition [
18,
21]. As a result, the enhanced upconversion red emission greatly improved the PDT effect on periodontal disease. The Ce6 loaded UCNPs composites with efficient red upconversion luminescence could be potentially employed for aPDT applications.
3. Results and Discussions
The schematic
Figure 1 illustrates the synthesis of UCNPs@Ce6@silane composites. The Ce6 molecules have hydrophobic nature and are difficult to be introduced inside the in vivo environment. In this case, the Ce6 molecules were loaded and coated with a very thin silane layer with a thickness of 2–3 nm. When the upconversion nanocomposite is endocytosed by bacterial, the UCNPs can emit green and red light under the excitation of 980 nm NIR light, and the Ce6 molecules within the hydrophobic layer can be excited by the upconversion red emission, performing the aPDT function. The singlet oxygen is highly cytotoxic and can efficiently damage a variety of biomolecules, such as protein, nucleic acids and lipids, and in this design the Ce6 molecules can be well encapsulated and triggered only in the infectious area.
As shown in
Figure 2A,B the synthesized UCNPs and the silane modified UCNPs were characterized by TEM. It can be seen that the size distribution of both NPs is very uniform, about 25 nm for UCNPs and 30 nm for silane modified UCNPs. After silane coating, a thin layer of silane can be seen on the surface of the particles with a thickness of about 2–3 nm, as the black arrows point in
Figure 2B. When a single nanoparticle is selected and characterized by high-power transmission electron microscopy, the thin layer of silane on the surface can be clearly examined, as indicated by the white arrow. The FTIR spectra before and after silane coating were shown in
Figure 2C. It can be seen that, after silane modification with 18 carbon atoms chain, the characteristic peaks of Si-O-Si and Si-OH appear new compared to the unmodified UCNPs, indicating the successful modification of silane. Moreover, the intensity of the characteristic peak of -CH
2 increases relative to the unmodified UCNPs due to the long carbon chain, which is also consistent with the experimental conditions. The zeta potential of NaYF
4:Yb
3+,Er
3+@Ce6@silane and samples with the Mn doping percent of 10%, 20% and 30% composites are −28.5 mV, −27.8 mV, −33.5 mV, and −32.8 mV, respectively, indicating the high water solubility and stability.
It should be noted that, in addition to the complexity of the oral structure, the main difficulty of this NIR triggered PDT system lies in the design of the upconversion material. In some previous construction of photodynamic systems, photosensitizers were mostly supported on the nanocarriers by physical adsorption. For example, photosensitizer zinc phthalocyanine can be loaded into the mesopores of UCNP@mSiO
2 NPs [
19]. Though efficient production of
1O
2 was realized after these photosensitizers were loaded by physical adsorption, it still faces serious leakage problems. Therefore, the FRET efficiency becomes low in the PDT process, causing systemic toxic side effects as well. In this coating strategy, by focusing on improving the efficiency of photodynamic therapy, the UCNPs were modified with silane, achieving water solubility and biocompatibility. In addition, the stable and very thin coating layer can well encapsulate the Ce6 molecules by hydrophobic-hydrophobic interaction and the followed hydrolysis of the silane further form the close composite, avoiding the Ce6 leakage. Silanes of different carbon chain lengths were also tested. Considering the higher loading amount of Ce6 molecules, silane with 18 C atoms was employed as the coating layer.
The maximum amount of Ce6 molecules that can be loaded in the hydrophobic layer was investigated. Here, by fixing the amount of UCNPs and silane, the content of Ce6 was changed to test the stability. Herein the mass of the UCNPs was fixed at 8 mg, and the corresponding mass of Ce6 were changed from 300 to 1000 μg. In this case, the content of Ce6 molecules was not to exceed 800 μg or precipitation would occur, producing a flocculent precipitate due to the leakage of the hydrophobic molecules (data not shown).
The structure of UCNPs with and without Mn doping were measured by XRD as shown in
Figure 2D. Results confirmed the pure hexagonal phase NaYF
4 without Mn doping. After the Mn ions were introduced into the nanocrystals, the crystal structure of the UCNPs changes from a hexagonal to a cubic phase. In addition, as the Mn ions doping increased, it could be found that the diffraction peak of 111 plane shifted toward the large angle direction (shown in inset), further illustrating the success of Mn ions doping. Note that the Mn doping do not influence the morphology and size of the UCNPs.
The upconversion luminescent property of silane-coated UCNPs and Mn-doped UCNPs were investigated. Upconversion luminescence were obtained based on the anti-Stokes mechanism. The spectra of different Mn-doped samples were excited using a 980 nm continuous diode laser, where the laser power was adjusted to 1 W. From the upconversion emission spectra in
Figure 2E, the green emission was located at 528 and 546 nm, and red emission was located at 660 nm, corresponding to
2H
11/2,
4S
3/2 and
4F
9/2 excited states to the ground state
4I
15/2 transition of Er
3+, respectively. Yb
3+ ions serve as sensitizers which can absorb 980 nm photons more efficiently and then transfer energy to the activator Er
3+, thus completing the upconversion green and red emissions. Different Mn doped samples showed no change of the peak position in the emission spectrum, but the ratios of the green/red emissions, which is dependent on the Mn doping amount. The existence of Mn
2+ ions greatly influence the transition possibilities between green and red emissions of Er
3+. As the Mn doping content increases from 0 to 30%, the proportion of red light gradually increases. The fine-tuning of red/green emissions could be attributed to nonradiative energy transfer from the
2H
9/2 and
4S
3/2 levels of Er
3+ to the
4T
1 level of Mn
2+, followed by back-energy transfer to the
4F
9/2 level of Er
3+ as shown in
Figure 2F, resulting in an enhanced red emission output by rational controlling the Mn
2+ doping level. The energy transfer from level
4S
3/2 to the Mn ion can be proved by the lifetime since the occurrence of such non-radiative transitions will reduce lifetime value. Relative to the NaYF
4:Yb
3+,Er
3+@Ce6@silane UCNPs, all of the Mn doped UCNPs show the decreased lifetime in the green emission energy level. In addition, as the Mn doping content increases, the lifetime decreases gradually, and due to that the non-radiative transition speed is much faster than the radiation transition, as shown in the inset in
Figure 2E, indicating that the more efficient energy transfer to the Mn ion happens, thus causing the further enhancement of red emission. It should be noted that this luminescence property is beneficial for the Ce6 based aPDT, because the excitation of the Ce6 molecule is located in the red region, and, in addition, the Ce6 molecule is on the surface of the UCNPs with a very close distance, thus facilitating the upconversion aPDT.
The absorption spectrum of the Ce6 molecule inside the composites and the upconversion fluorescence spectrum of Mn 30% doped UCNPs are shown in
Figure 3A. The absorption at the red region is the primary excitation band of the Ce6 molecule for singlet oxygen production and this region is totally overlapped with the upconversion red emission band of its UCNPs carrier. Therefore, the enhanced upconversion red emission can further improve the aPDT effect. In addition, since the Ce6 molecule is located in the hydrophilic thin layer of the NPs, this energy transfer is very efficient for stimulating the PDT function. In the present study, up to 30% Mn was selected to dope into UCNPs to realize the enhancement of red light emission. Too much foreign element doping would lead to the change of crystal lattice, following with the functional variation of UCNPs. Besides, in this doping range (10–30%), the fluorescence intensity of red light increases with Mn doping, while the overall fluorescence intensity also decreases as the Mn content further increases. Previous studies have demonstrated that the introduction of sufficient Mn
2+ ions into NaYF
4:Yb/Er leads to a brilliant red emission, which is brighter by as much as 15 folds than that of Mn-free sample [
25]. Therefore, in this doping scale, the highest red emission intensity was obtained, which is better for the efficacy of aPDT. In addition, in such doping situation, green light was also retained, which can be further used for fluorescence imaging. Currently, the up-conversion green light as imaging signal and red light as PDT treatment source need to be further investigated.
The upconversion PDT function of composite nanomaterials was tested using the singlet oxygen probe ABDA 9,10-fluorenyl-di(methylene)dimalonic acid [
26].
Figure 3B shows the absorption spectra of magnetic nanocomposites under red light with an interval of 2 min. As the irradiation time increases, the absorption value of ABDA at 260 nm is reduced, indicating the generation of singlet oxygen. Furthermore, the absorption band at 400 nm showed the similar tendency, because the Ce6 molecules can also be consumed via the photodegradation of red light. It should be noted here that the detection efficiency of singlet oxygen is not very high according to the probe absorption because of the fact that the 980 nm excitation area is usually small, while the diffusion region of singlet oxygen is relatively large in the solution. This test proves that the composite material can produce singlet oxygen, indicating the successful material design and preparation. For periodontal disease treatments, the small 980 nm laser irradiation area is enough for sterilization. Dark cytotoxicity of NaYF
4:Yb
3+,Er
3+@Ce6@silane NPs was investigated with L929 mouse fibroblast cells by CCK-8 assay, as shown in
Figure 3C. The UCNPs show very good biocompatibility. The results showed that the cells were still 90% viable with the concentration of 200 μg/mL, indicating very low cytotoxicity. In this study, it should be attributed to the silane modification and the negatively-charged surface which could reduce cytotoxicity, showing great potential for new photosensitizer carriers in dental application.
Upconversion red light triggered PDT was first tested within biofilm experiments. The sample was irradiated with a 980 nm continuous diode laser which was adjusted to 750 J·cm
−2 by tuning irradiation area for 3 min. Representative results of the live/dead analysis were performed and the results are shown in
Figure 4 P. gingivalis, P. intermedia and
F. nucleatum were selected as the bacteriostatic models in this work simulating early, middle and late stages of biofilm development, respectively, and colonized in plaque biofilms [
27]. Live bacteria were stained as green which mainly in the control group and dead bacteria were stained red. In all three kinds of bacteria, NaYF
4@Ce6@silane plays an efficient role in aPDT function. There are more and more dead bacteria in the groups with NaYF
4@Ce6@silane and Mn doped NaYF
4@Ce6@silane NPs under 980 nm light irradiation. The red color increases as the Mn doping increases, due to the enhanced upconversion red emission. The corresponding enhanced aPDT from Ce6 caused more and more dead bacteria. Note that the power density of the laser used in this study is strong enough for application of cell level in vitro. In addition, though the proposed periodontal bacteria locate in deepest periodontal pockets, usually 5–8 mm from the gingival margins, the power of the irradiation laser can still reach the threshold value. It is reported that the 980 nm laser can penetrate more than 0.7 cm in pork tissue without obvious reduction of power density [
17,
19,
28].
Several kinds of UCNPs were applied to covert NIR to visible light or UV light for triggering PDT in tumor therapy or antibacterial application [
29,
30,
31]. Gulzar et al. synthesized a nanocomposite based on nanographene oxide-UCNPs-Ce6 as a theranostic platform for the upconversion luminescence imaging-guided PDT/PTT of cancer [
29]. The tremendous surface area of graphene oxide was allowed to house Ce6, as well as UCNPs. Remarkably, both the imaging and dual-mode treatments in this nanoplatform are stimulated by light, which unveils outstanding gains in terms of augmenting cancer killing specificity and decreasing side effects [
29]. Numerous UCNPs-based nanomaterials with varieties of structures for photodynamic therapy in cancer treatment were summarized in a recent study [
32]. On the other hand, for antibacterial application, Zhang et al. developed a photosensitizer (β-carboxyphthalocyanine zinc, CPZ) delivery system with UCNPs (LiYF
4:Yb/Er) and polyvinylpyrrolidone (PVP) [
30]. Such a near-infrared (NIR) triggered UCNPs-CPZ-PVP system significantly reduced the aggregation of CPZ and presented a high anti-infectious activity against multi-drug resistant bacteria (methicillin-resistant
Staphylococcus aureus by 4.7 log and multi-drug resistant
Escherichia coli by 2.1 log). Another study investigated the dual antibacterial behavior induced by the curcumin-UCNPs itself and induced by photodynamic therapy were demonstrated [
31]. The results showed that nearly 100% methicillin-resistant
Staphylococcus aureus was eradicated using curcumin-UCNPs under the NIR irradiation. However, to the best of our knowledge, the present study is the first report on application of near-infrared light to achieve photodynamic therapy for periodontitis treatment. We combine the upconversion luminescent material and the photosensitizer so that the near-infrared light with high tissue penetration depth can be utilized. More importantly, traditional aPDT had a minimal effect on the viability of microorganisms organized in a bacterial biofilm, which was probably due to the hydrophobic nature of the most photosensitizer molecules, leading to the reduced penetration of the photosensitizer into the biofilm matrix. The present study developed a silane coating as the shell of the UCNPs and embedded Ce6 inside the thin layer. This design would improve the hydrophilicity of the nanoparticles, and thus overcome the drainage of gingival crevicular fluid and high saliva fluid turnover [
24]. Furthermore, the energy transfer would be more efficient for aPDT triggering due to the existence of Ce6 in the hydrophilic thin layer of the NPs. Therefore, this is a new exploration for the treatment of oral periodontitis, which is of great significance.
There are two possible aPDT mechanisms which can be described as follows: The triplet state photosensitizer Ce6 either can undergo a type I reaction, or a type II reaction. Type I: excited triplet Ce6 reacts directly with the macromolecule (protein, nucleic acid, lipids, etc.), generating free radicals or free radical ions by electron transfer, and further react with oxygen molecules, forming highly reactive oxides such as hydroxyl radicals, peroxides, etc. Type II: triplet photosensitizer Ce6 react with surrounding ground state oxygen molecules, generating singlet oxygen, which has ultrahigh cytotoxicity by oxidation and peroxidation of the cellular structure, microbial attack, destruction of the cell wall, and membrane system damage, thus affecting microbial metabolism and leading to cell death [
33,
34].
In this work, NaYF
4@Ce6@silane nanocrystals release singlet oxygen through a type II reaction, which can penetrate into plaque and subsequently kill bacteria. Note that the nanosized material can enter the microorganisms by endocytosis which has been proven in previous studies [
35,
36,
37]. In the present study, the high permeability of silane modified UCNPs are responsible for the efficient reactive oxygen generation and PDT effect.
Regarding the periodontitis treatment, aPDT that produces ROS possibly would not exacerbate the inflammatory response due to the following reasons: First, the role of PDT in inflammation is a complex process. Indeed, in tumor therapy, PDT could produce a strong inflammatory response of infiltration with neutrophils, mast cells, lymphocytes, monocytes, and macrophages [
38]. However, PDT could also increase the stability of interleukin 10 (IL-10) RNA and/or increase the transcription efficiency of IL-10, which is an anti-inflammatory cytokine that inhibited cell-mediated immune responses [
39]. Gollnick
et al [
40] reported that PDT could change the activity of a gene promoter and increase the expression level of IL-10. Therefore, these two processes may co-exist in the PDT therapy. Second, in general,
1O
2 diffusion distance is only about 100 nm, and the half-life is <0.04 μs [
41], the photosensitizer should be precisely delivered to the area of periodontal disease. Hence, the distance of
1O
2 diffusion to the bacterial cells is of significant importance for the aPDT activity. Henderson et al. proposed that the
1O
2-induced photodamage from porphyrin activation is usually localized to within 0.1 µm of its site of release [
42]. Therefore, when the sterilization process is completed, the energy will disappear and the inflammation promotion effect on normal tissues is limited. These should be the main reasons for the efficient antibacterial properties, but without obvious inflammation. Alternatively, it is a promising approach to incorporation of anti-inflammatory agents (such as ceria, etc.) into the design of nanoparticles for aPDT application to reduce the potential risks in the future [
43]. Considering the complicated oral structure and infectious area is always in deep tissue, these 980 nm laser excited UCNPs with large penetration depth are the most suitable photosensitizer carriers and energy transfer donors for antibacterial application. In addition, the Ce6 molecules are located on the surface of the UCNPs within very close distance because of the very thin silane-modified layer, forming the very close excitation distance. Therefore, besides the enhanced upconversion red light emission from NaYF
4 UCNPs, efficient
1O
2 production can be obtained for the antibacterial action against periodontal pathogens.
The CFU assay is the most essential for evaluating a new antimicrobial method. In this work, the CFU of 4-day biofilms of (A)
P. gingivalis, (B)
P. intermedia and (C)
F. nucleatum after aPDT were measured and shown in
Figure 5. In aPDT treatments, the biofilm matrix was easily disrupted with a deeper penetrated infrared light. The value for single-strain biofilms on dentin is different among the samples treated with different Mn doped NaYF
4@Ce6@silane NPs. The control group shows the highest CFU value. After the treatment with the NPs and the 980 nm irradiation, the CFU experienced significant reductions for all three species compared to the control groups (
p < 0.05). Among different bacterial species, NaYF
4@Ce6@silane and NaYF
4-Mn10%@Ce6@silane groups show similar CFU results, while, as Mn doping increases, the CFU values further decreases. A similar trend was observed among all the three bacterial species with the same aPDT procedure, indicating the universality of this upconversion aPDT agent. The three bacterial CFU counts of 4-day biofilms all showed a logarithmic reduction of more than 2 log with the Mn30% doped NPs. This high efficacy against periodontitis-related biofilms should be attributed to the high hydrophilic surface after silane modification, as well as the upconversion luminescence triggered aPDT.
It is known that, relative to planktonic bacteria, biofilms are much more difficult to kill because of the formation of extracellular polymeric substance inside the biofilm which greatly resist the entry of conventional antibacterial agents [
44]. In such a situation, the singlet oxygen from PDT can play an important function due to its efficient diffusion and the oxidation of amino acids and DNA damage, while the aPDT agent carrier should be located in part of the disease. In this work, we further involved the upconversion aPDT with deep tissue penetration, which can solve the problem of bacterial treatment in deep periodontal tissue.
The polysaccharide production of the biofilms was measured because polysaccharide is produced by live bacteria and then related to bacterial viability. Extracellular polymeric substance (EPS) protects pathogens from antibacterial agents and contributes to the virulence and pathogenicity of pathogens via small molecule mediated inter- and intra- species crosstalk. It is mainly composed of polysaccharides, proteins and extracellular DNA and accounts for about 90% of the total mass of the biofilm [
45]. EPS with polysaccharide can be regulated by external stimuli since most glycoproteins are located on the outer membrane of Gram-negative bacteria. The polysaccharide production results of biofilms of (A)
P. gingivalis, (B)
P. intermedia and (C)
F. nucleatum on dentin squares are plotted in
Figure 6. For each species, the control group without UCNPs shows similar polysaccharide amounts (
p > 0.1). The polysaccharide production for all three species was greatly reduced with the addition of NaYF
4-Mn@Ce6@silane NPs under 980 nm irradiation compared to the control group (
p < 0.05). These would probably be co-related to the decreased number of bacteria after aPDT treatments. Also, since the increased Mn doping enhanced the upconversion red emission and then aPDT function, the polysaccharide production decreased with the increasing Mn doping concentration from 0% to 30%. Therefore, the reduction in EPS via aPDT could reduce/destroy the protection of all three species of the bacteria.
Clinically, the subgingival biofilm is an aggregation of multispecies bacteria. Multispecies biofilms are more challenging to eradicate than single species biofilms and planktonic bacteria [
46]. The early attached dominant species of bacteria are streptococci and members of the yellow and purple complexes, such as
Actinomycess pp. which soon develop a polymicrobial community [
47]. However, most studies also have been done in planktonic or single-species biofilms without regard for the complex microbial and biochemical changes occurring simultaneously [
24]. Besides the standardized simple model for culturing in vitro, intact single-species biofilms were easy to realize in a normal laboratory. Within the limitations of this in vitro study, the biofilm model gave a simple means of determining the antimicrobial efficacy of novel nanocomposite with Mn doping upon NIR irradiation against three key periodontal pathogens. This methodology may be more clinically representative than the methods, which do not consider the microorganism in biofilms [
48]. However, it still does not reproduce what happens clinically in the periodontal pocket. In such an environment, several mechanisms allow the growth and selection of several microorganisms, even after the treatment. Therefore, further studies should focus on the susceptibility of novel nanocomposite based aPDT against the multispecies biofilms and confirm the effects in animal studies.