Magnetized Carbon Nanotube Based Lateral Flow Immunoassay for Visual Detection of Complement Factor B
Abstract
:1. Introduction
2. Results and Discussion
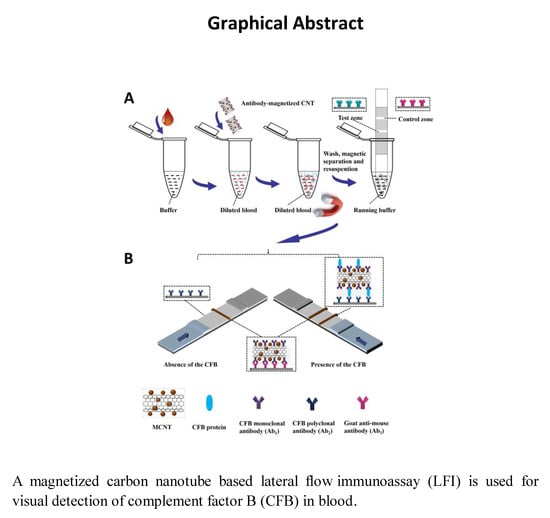
2.1. Principle of MCNT-Based LFI for CFB Detection
2.2. Parameters Optimization
2.3. LFI Performance Comparison of Monoclonal or Polyclonal Antibody as Detection Antibody
2.4. Analytical Performance in Flow Buffer
2.5. Analysis of CFB in Blood
2.6. Comparison of Analytical Performance of MCNT-based LFI and Commercial CFB ELISA Kit.
3. Experimental Section
3.1. Reagents and Chemicals
3.2. Apparatus
3.3. Preparation of Magnetized Carbon Nanotube (MCNTs) and Gold Nanoparticle (GNPs)
3.4. Preparation of the MCNT-Ab1, MCNT-Ab2 and GNP-Ab1 Conjugates
3.5. Preparation of Lateral Flow Test Strips
3.6. Assay Procedure
3.6.1. CFB Detection in Buffer
3.6.2. CFB Detection in Whole Blood
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, B.P.; Harris, C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 2015, 14, 857–877. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, M.J.; Sughrue, M.E.; Kane, A.J.; Mills, S.A.; Parsa, A.T. Cancer and the complement cascade. Mol. Cancer Res. 2010, 8, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Na, K.; Jeong, S.K.; Lim, J.S.; Kim, S.A.; Lee, M.J.; Song, S.Y.; Kim, H.; Hancock, W.S.; Paik, Y.K. Identification of human complement factor B as a novel biomarker candidate for pancreatic ductal adenocarcinoma. J. Proteome Res. 2014, 13, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea de Jorge, E.; Harris, C.L.; Esparza-Gordillo, J.; Carreras, L.; Arranz, E.A.; Garrido, C.A.; Lopez-Trascasa, M.; Sanchez-Corral, P.; Morgan, B.P.; Rodriguez de Cordoba, S. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.; Prassas, I.; Dimitromanolakis, A.; Brand, R.E.; Serra, S.; Diamandis, E.P.; Blasutig, I.M. Validation of biomarkers that complement CA19.9 in detecting early pancreatic cancer. Clin. Cancer Res. 2014, 20, 5787–5795. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsson, J.; Larsson, A.; Sjoholm, A.G.; Truedsson, L.; Jansson, B.; Borrebaeck, C.A.; Wingren, C. Design of recombinant antibody microarrays for serum protein profiling: targeting of complement proteins. J. Proteome Res. 2007, 6, 3527–3536. [Google Scholar] [CrossRef]
- Andoh, A.; Fujiyama, Y.; Sakumoto, H.; Uchihara, H.; Kimura, T.; Koyama, S.; Bamba, T. Detection of complement C3 and factor B gene expression in normal colorectal mucosa, adenomas and carcinomas. Clin. Exp. Immunol. 1998, 111, 477–483. [Google Scholar] [CrossRef]
- Ünlü, M.; de Lange, R.P.J.; de Silva, R.; Kalaria, R.; St. Clair, D. Detection of complement factor B in the cerebrospinal fluid of patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy disease using two-dimensional gel electrophoresis and mass spectrometry. Neurosci. Lett. 2000, 282, 149–152. [Google Scholar]
- Chan, A.; Diamandis, E.P.; Blasutig, I.M. Strategies for discovering novel pancreatic cancer biomarkers. J. Proteom. 2013, 81, 126–134. [Google Scholar] [CrossRef]
- Wu, J.; Xie, X.; Liu, Y.; He, J.; Benitez, R.; Buckanovich, R.J.; Lubman, D.M. Identification and confirmation of differentially expressed fucosylated glycoproteins in the serum of ovarian cancer patients using a lectin array and LC-MS/MS. J. Proteome Res. 2012, 11, 4541–4552. [Google Scholar] [CrossRef]
- Mao, X.; Ma, Y.; Zhang, A.; Zhang, L.; Zeng, L.; Liu, G. Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal. Chem. 2009, 81, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, H.; Baloda, M.; Gurung, A.S.; Xu, L.-P.; Wang, T.; Zhang, X.; Liu, G. Visual detection of microRNA with lateral flow nucleic acid biosensor. Biosens. Bioelectro. 2014, 54, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, J.; Birrenkott, J.; Zhao, J.X.; Takalkar, S.; Baryeh, K.; Liu, G. Gold-nanoparticle-decorated silica nanorods for sensitive visual detection of proteins. Anal. Chem. 2014, 86, 7351–7359. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Merkoci, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mao, X.; Zeng, Q.; Wang, S.; Kawde, A.N.; Liu, G. Aptamer-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for protein analysis. Anal. Chem. 2009, 81, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Spano, G.; Speranskaya, E.S.; Goryacheva, I.Y.; Baggiani, C. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim. Acta 2018, 185, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Fan, K.; Zhang, D.; Zhang, J.; Tan, S.; Liang, M.; Liu, Y.; Zhang, P.; Liu, W.; Qiu, X.; et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Takalkar, S.; Baryeh, K.; Liu, G. Fluorescent carbon nanoparticle-based lateral flow biosensor for ultrasensitive detection of DNA. Biosens. Bioelectron. 2017, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, Y.; Baryeh, K.; Takalkar, S.; Lund, M.; Zhang, X.; Liu, G. Magnetized Carbon Nanotubes for Visual Detection of Proteins Directly in Whole Blood. Anal. Chim. Acta 2017, 993, 79–86. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, Y.; Baryeh, K.; Takalkar, S.; Lund, M.; Zhang, X.; Liu, G. Lateral flow assay for carbohydrate antigen 19-9 in whole blood by using magnetized carbon nanotubes. Microchim. Acta 2017, 184, 4287–4294. [Google Scholar] [CrossRef]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs. polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Inter. 2012, 4, 6949–6956. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yoon, H. Fabrication of Magnetic Carbon Nanotubes Using a Metal-Impregnated Polymer Precursor. Adv. Mater. 2003, 15, 2088–2091. [Google Scholar] [CrossRef]
- Rey, E.; O’Dell, D.; Mehta, S.; Erickson, D. Mitigating the Hook Effect in Lateral Flow Sandwich Immunoassays Using Real-Time Reaction Kinetics. Anal. Chem. 2017, 89, 5095–5100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: not available. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wu, T.; Wang, F.; Li, K.; Qian, L.; Zhang, X.; Liu, G. Magnetized Carbon Nanotube Based Lateral Flow Immunoassay for Visual Detection of Complement Factor B. Molecules 2019, 24, 2759. https://doi.org/10.3390/molecules24152759
Huang Y, Wu T, Wang F, Li K, Qian L, Zhang X, Liu G. Magnetized Carbon Nanotube Based Lateral Flow Immunoassay for Visual Detection of Complement Factor B. Molecules. 2019; 24(15):2759. https://doi.org/10.3390/molecules24152759
Chicago/Turabian StyleHuang, Yan, Tingting Wu, Fang Wang, Kun Li, Lisheng Qian, Xueji Zhang, and Guodong Liu. 2019. "Magnetized Carbon Nanotube Based Lateral Flow Immunoassay for Visual Detection of Complement Factor B" Molecules 24, no. 15: 2759. https://doi.org/10.3390/molecules24152759
APA StyleHuang, Y., Wu, T., Wang, F., Li, K., Qian, L., Zhang, X., & Liu, G. (2019). Magnetized Carbon Nanotube Based Lateral Flow Immunoassay for Visual Detection of Complement Factor B. Molecules, 24(15), 2759. https://doi.org/10.3390/molecules24152759