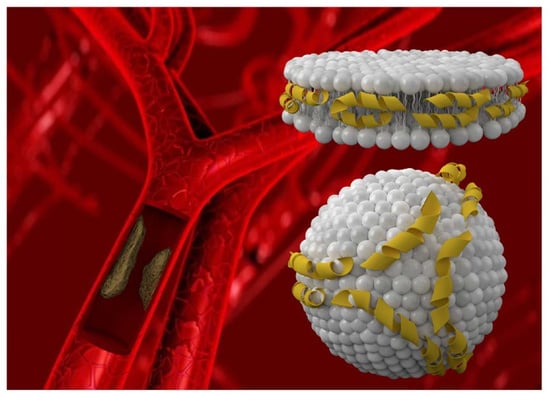
Artificial High Density Lipoprotein Nanoparticles in Cardiovascular Research
Abstract
:1. Lipoprotein Metabolism and Coronary Heart Disease
2. Synthesis of rHDL Nanoparticles
3. Structural Aspects of apo-AI Mimetic Peptides
4. Physicochemical Characterization of rHDL
5. rHDL-Based Therapy in CVDs
6. rHDL as Delivery Systems in Therapy and Diagnosis of CVDs
7. Apo-AI Mimetics in the Therapy of CVDs
8. rHDL Apo-AI Mimetics in the Therapy of CVDs
9. Challenges, Advantages, and Limitations
10. Conclusion and Perspectives
Funding
Conflicts of Interest
Abbreviations
| HDL | high density lipoprotein |
| rHDL | reconstituted high density lipoprotein |
| CHD | coronary heart disease |
| CVD | cardiovascular disease |
| RCT | reverse cholesterol transport |
| apo-AI apo-E | apolipoprotein AI apolipoprotein E |
| ABCA1 | ATP binding cassette transporter A1 |
| LCAT | lecithin cholesterol acyl transferase |
| CETP | cholesterol ester transfer proteins |
| VLDL | very low density lipoprotein |
| LDL | low density lipoprotein |
| SR-B1 | scavenger receptor class B type I |
| DMPC | dimyristoyl-phosphatidylcholine |
| POPC | palmitoyl-oleoly-phosphatidylcholine |
| DPPC | dipalmitoyl-phosphatidylcholine |
| PS | phosphatidylserine |
| SM | sphingomyelin |
| DPPG | dipalmitoyl-sn-glycero-3-phosphorylglycerol |
| CT | computer tomography |
| MRI | magnetic resonance imaging |
| DLS | dynamic light scattering |
| NTA | nanoparticle tracking analysis |
| TEM | transmission electron microscopy |
| AFM | atomic force microscopy |
| MPS | mononuclear phagocytic system |
| DMPE-DTPA | 1,2 di-myristoyl-sn-glycero-3-phosphatidylethanolamine–diethylenetriamine pentaacetic acid |
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.J.; Fielding, P.E. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995, 36, 211–228. [Google Scholar] [PubMed]
- Von Eckardstein, A.; Nofer, J.R.; Assmann, G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Pourmousa, M.; Song, H.D.; He, Y.; Heinecke, J.W.; Segrest, J.P.; Pastor, R.W. Tertiary structure of apolipoprotein A-I in nascent high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2018, 115, 5163–5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segrest, J.P.; Jones, M.K.; Klon, A.E.; Sheldahl, C.J.; Hellinger, M.; De Loof, H.; Harvey, S.C. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J. Biol. Chem. 1999, 274, 31755–31758. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; Huang, R.; Morris, J.; Fang, J.; Gracheva, E.O.; Ren, G.; Kontush, A.; Jerome, W.G.; Rye, K.A.; Davidson, W.S. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. USA 2008, 105, 12176–12181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Smith, J.D. Insight into ABCG1-mediated cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1198–1200. [Google Scholar] [CrossRef]
- Michell, D.L.; Vickers, K.C. HDL and microRNA therapeutics in cardiovascular disease. Pharmacol. Ther. 2016, 168, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, K.C.; Remaley, A.T. Lipid-based carriers of microRNAs and intercellular communication. Curr. Opin. Lipidol. 2012, 23, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Tan, L.; Long, J.L.; Davidson, W.S. Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013, 54, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Silva, R.A.; Chantepie, S.; Lagor, W.R.; Chapman, M.J.; Kontush, A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Balady, G.J.; Criqui, M.H.; Fletcher, G.; Greenland, P.; Hiratzka, L.F.; Houston-Miller, N.; Kris-Etherton, P.; Krumholz, H.M.; LaRosa, J.; et al. Primary prevention of coronary heart disease: Guidance from Framingham: A statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation 1998, 97, 1876–1887. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Chapman, M.J.; Fazio, S.; Hussain, M.M.; Kontush, A.; Krauss, R.M.; Otvos, J.D.; Remaley, A.T.; Schaefer, E.J. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011, 57, 392–410. [Google Scholar] [CrossRef]
- Farrer, S. Beyond Statins: Emerging Evidence for HDL-Increasing Therapies and Diet in Treating Cardiovascular Disease. Adv. Prev. Med. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Linsel-Nitschke, P.; Tall, A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 2005, 4, 193–205. [Google Scholar] [CrossRef]
- Du, X.M.; Kim, M.J.; Hou, L.; Le Goff, W.; Chapman, M.J.; Van Eck, M.; Curtiss, L.K.; Burnett, J.R.; Cartland, S.P.; Quinn, C.M.; et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 2015, 116, 1133–1142. [Google Scholar] [CrossRef]
- Zakiev, E.; Rached, F.; Lhomme, M.; Darabi-Amin, M.; Ponnaiah, M.; Becker, P.H.; Therond, P.; Serrano, C.V., Jr.; Santos, R.D.; Chapman, M.J.; et al. Distinct phospholipid and sphingolipid species are linked to altered HDL function in apolipoprotein A-I deficiency. J. Clin. Lipid. 2019, 13, 468–480. [Google Scholar] [CrossRef]
- Bricarello, D.A.; Smilowitz, J.T.; Zivkovic, A.M.; German, J.B.; Parikh, A.N. Reconstituted lipoprotein: A versatile class of biologically-inspired nanostructures. ACS Nano 2011, 5, 42–57. [Google Scholar] [CrossRef]
- Tsujita, M.; Wolska, A.; Gutmann, D.A.P.; Remaley, A.T. Reconstituted Discoidal High-Density Lipoproteins: Bioinspired Nanodiscs with Many Unexpected Applications. Curr. Atheroscler. Rep. 2018, 20, 59. [Google Scholar] [CrossRef]
- Melchior, J.T.; Walker, R.G.; Cooke, A.L.; Morris, J.; Castleberry, M.; Thompson, T.B.; Jones, M.K.; Song, H.D.; Rye, K.A.; Oda, M.N.; et al. A consensus model of human apolipoprotein A-I in its monomeric and lipid-free state. Nat. Struct. Mol. Biol. 2017, 24, 1093–1099. [Google Scholar] [CrossRef]
- Luthi, A.J.; Patel, P.C.; Ko, C.H.; Mutharasan, R.K.; Mirkin, C.A.; Thaxton, C.S. Nanotechnology for synthetic high-density lipoproteins. Trends Mol. Med. 2010, 16, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Damiano, M.G.; Mutharasan, R.K.; Tripathy, S.; McMahon, K.M.; Thaxton, C.S. Templated high density lipoprotein nanoparticles as potential therapies and for molecular delivery. Adv. Drug Deliv. Rev. 2013, 65, 649–662. [Google Scholar] [CrossRef]
- Thaxton, C.S.; Rink, J.S.; Naha, P.C.; Cormode, D.P. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 116–131. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, J.B. Evaluation of reconstituted high-density lipoprotein (rHDL) as a drug delivery platform—A detailed survey of rHDL particles ranging from biophysical properties to clinical implications. Nanomedicine 2016, 12, 2161–2179. [Google Scholar] [CrossRef]
- Kingwell, B.A.; Chapman, M.J.; Kontush, A.; Miller, N.E. HDL-targeted therapies: Progress, failures and future. Nat. Rev. Drug Discov. 2014, 13, 445–464. [Google Scholar] [CrossRef]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; van Lenten, B.J.; Fogelman, A.M. HDL as a biomarker, potential therapeutic target, and therapy. Diabetes 2009, 58, 2711–2717. [Google Scholar] [CrossRef]
- Navab, M.; Shechter, I.; Anantharamaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 164–168. [Google Scholar] [CrossRef]
- Van Lenten, B.J.; Wagner, A.C.; Anantharamaiah, G.M.; Navab, M.; Reddy, S.T.; Buga, G.M.; Fogelman, A.M. Apolipoprotein A-I mimetic peptides. Curr. Atheroscler. Rep. 2009, 11, 52–57. [Google Scholar] [CrossRef]
- Hovingh, G.K.; Bochem, A.E.; Kastelein, J.J. Apolipoprotein A-I mimetic peptides. Curr. Opin. Lipidol. 2010, 21, 481–486. [Google Scholar] [CrossRef]
- Almer, G.; Mangge, H.; Zimmer, A.; Prassl, R. Lipoprotein-Related and Apolipoprotein-Mediated Delivery Systems for Drug Targeting and Imaging. Curr. Med. Chem. 2015, 22, 3631–3651. [Google Scholar] [CrossRef]
- Mallory, J.B.; Kushner, P.J.; Protter, A.A.; Cofer, C.L.; Appleby, V.L.; Lau, K.; Schilling, J.W.; Vigne, J.L. Expression and characterization of human apolipoprotein A-I in Chinese hamster ovary cells. J. Biol. Chem. 1987, 262, 4241–4247. [Google Scholar]
- Pyle, L.E.; Sawyer, W.H.; Fujiwara, Y.; Mitchell, A.; Fidge, N.H. Structural and functional properties of full-length and truncated human proapolipoprotein AI expressed in escherichia coli. Biochemistry 1996, 35, 12046–12052. [Google Scholar] [CrossRef]
- Massey, J.B.; Pownall, H.J. Cholesterol is a determinant of the structures of discoidal high density lipoproteins formed by the solubilization of phospholipid membranes by apolipoprotein A-I. Biochim. Biophys. Acta 2008, 1781, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Pittman, R.C.; Glass, C.K.; Atkinson, D.; Small, D.M. Synthetic high density lipoprotein particles. Application to studies of the apoprotein specificity for selective uptake of cholesterol esters. J. Biol. Chem. 1987, 262, 2435–2442. [Google Scholar]
- Jonas, A. Reconstitution of High-Density Lipoproteins. Methods Enzymol. 1986, 128, 553–582. [Google Scholar]
- Miyazaki, M.; Tajima, Y.; Ishihama, Y.; Handa, T.; Nakano, M. Effect of phospholipid composition on discoidal HDL formation. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 1340–1346. [Google Scholar] [CrossRef] [Green Version]
- Chromy, B.A.; Arroyo, E.; Blanchette, C.D.; Bench, G.; Benner, H.; Cappuccio, J.A.; Coleman, M.A.; Henderson, P.T.; Hinz, A.K.; Kuhn, E.A.; et al. Different apolipoproteins impact nanolipoprotein particle formation. J. Am. Chem. Soc. 2007, 129, 14348–14354. [Google Scholar] [CrossRef]
- Murray, S.C.; Gillard, B.K.; Ludtke, S.J.; Pownall, H.J. Direct Measurement of the Structure of Reconstituted High-Density Lipoproteins by Cryo-EM. Biophys. J. 2016, 110, 810–816. [Google Scholar] [CrossRef]
- Cukier, A.M.O.; Therond, P.; Didichenko, S.A.; Guillas, I.; Chapman, M.J.; Wright, S.D.; Kontush, A. Structure-function relationships in reconstituted HDL: Focus on antioxidative activity and cholesterol efflux capacity. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2017, 1862, 890–900. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Therond, P.; Zerrad, A.; Couturier, M.; Negre-Salvayre, A.; de Souza, J.A.; Chantepie, S.; Chapman, M.J. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: Relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1843–1849. [Google Scholar] [CrossRef]
- Darabi, M.; Guillas-Baudouin, I.; Le Goff, W.; Chapman, M.J.; Kontush, A. Therapeutic applications of reconstituted HDL: When structure meets function. Pharmacol. Ther. 2016, 157, 28–42. [Google Scholar] [CrossRef]
- Beck, W.H.; Adams, C.P.; Biglang-Awa, I.M.; Patel, A.B.; Vincent, H.; Haas-Stapleton, E.J.; Weers, P.M. Apolipoprotein A-I binding to anionic vesicles and lipopolysaccharides: Role for lysine residues in antimicrobial properties. Biochim. Biophys. Acta. 2013, 1828, 1503–1510. [Google Scholar] [CrossRef]
- de Beer, M.C.; Durbin, D.M.; Cai, L.; Jonas, A.; de Beer, F.C.; van der Westhuyzen, D.R. Apolipoprotein A-I conformation markedly influences HDL interaction with scavenger receptor BI. J. Lipid Res. 2001, 42, 309–313. [Google Scholar]
- Kim, Y.; Fay, F.; Cormode, D.P.; Sanchez-Gaytan, B.L.; Tang, J.; Hennessy, E.J.; Ma, M.; Moore, K.; Farokhzad, O.C.; Fisher, E.A.; et al. Single step reconstitution of multifunctional high-density lipoprotein-derived nanomaterials using microfluidics. ACS Nano 2013, 7, 9975–9983. [Google Scholar] [CrossRef]
- Sanchez-Gaytan, B.L.; Fay, F.; Lobatto, M.E.; Tang, J.; Ouimet, M.; Kim, Y.; van der Staay, S.E.; van Rijs, S.M.; Priem, B.; Zhang, L.; et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjugate Chem. 2015, 26, 443–451. [Google Scholar] [CrossRef]
- Tang, J.; Baxter, S.; Menon, A.; Alaarg, A.; Sanchez-Gaytan, B.L.; Fay, F.; Zhao, Y.; Ouimet, M.; Braza, M.S.; Longo, V.A.; et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl. Acad. Sci. USA 2016, 113, E6731–E6740. [Google Scholar] [CrossRef] [Green Version]
- Borhani, D.W.; Engler, J.A.; Brouillette, C.G. Crystallization of truncated human apolipoprotein A-I in a novel conformation. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 1578–1583. [Google Scholar] [CrossRef]
- Segrest, J.P.; Jones, M.K.; DeLoof, H.; Brouillette, C.G.; Venkatachalapathi, Y.V.; Anantharamaiah, G.M. The amphipathic helix in the exchangeable apolipoproteins—A review of secondary structure and function. J. Lipid Res. 1992, 33, 141–166. [Google Scholar]
- Segrest, J.P.; de Loof, H.; Dohlman, J.G.; Brouillette, C.G.; Anantharamaiah, G.M. Amphipathic helix motif: Classes and properties. Proteins 1990, 8, 103–117. [Google Scholar] [CrossRef]
- Reddy, S.T.; Navab, M.; Anantharamaiah, G.M.; Fogelman, A.M. Apolipoprotein A-I mimetics. Curr. Opin. Lipidol. 2014, 25, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Datta, G.; Chaddha, M.; Hama, S.; Navab, M.; Fogelman, A.M.; Garber, D.W.; Mishra, V.K.; Epand, R.M.; Epand, R.F.; Lund-Katz, S.; et al. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J. Lipid Res. 2001, 42, 1096–1104. [Google Scholar]
- Epand, R.M.; Epand, R.F.; Sayer, B.G.; Melacini, G.; Palgulachari, M.N.; Segrest, J.P.; Anantharamaiah, G.M. An apolipoprotein AI mimetic peptide: Membrane interactions and the role of cholesterol. Biochemistry 2004, 43, 5073–5083. [Google Scholar] [CrossRef]
- Wool, G.D.; Reardon, C.A.; Getz, G.S. Apolipoprotein A-I mimetic peptide helix number and helix linker influence potentially anti-atherogenic properties. J. Lipid Res. 2008, 49, 1268–1283. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.K.; Palgunachari, M.N.; Lund-Katz, S.; Phillips, M.C.; Segrest, J.P.; Anantharamaiah, G.M. Effect of the arrangement of tandem repeating units of class A amphipathic alpha-helixes on lipid interaction. J. Biol. Chem. 1995, 270, 1602–1611. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Apolipoprotein A-I and A-I mimetic peptides: A role in atherosclerosis. J. Inflamm. Res. 2011, 4, 83–92. [Google Scholar] [CrossRef]
- Osei-Hwedieh, D.O.; Amar, M.; Sviridov, D.; Remaley, A.T. Apolipoprotein mimetic peptides: Mechanisms of action as anti-atherogenic agents. Pharmacol. Ther. 2011, 130, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Li, D.; Drake, L.; Yuan, W.; Deschaine, S.; Morin, E.E.; Ackermann, R.; Olsen, K.; Smith, D.E.; Schwendeman, A. Influence of route of administration and lipidation of apolipoprotein A-I peptide on pharmacokinetics and cholesterol mobilization. J. Lipid Res. 2017, 58, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Islam, R.M.; Pourmousa, M.; Sviridov, D.; Gordon, S.M.; Neufeld, E.B.; Freeman, L.A.; Perrin, B.S.; Pastor, R.W.; Remaley, A.T. Structural properties of apolipoprotein A-I mimetic peptides that promote ABCA1-dependent cholesterol efflux. Sci. Rep. 2018, 8, 2956. [Google Scholar] [CrossRef]
- D’Souza, W.; Stonik, J.A.; Murphy, A.; Demosky, S.J.; Sethi, A.A.; Moore, X.L.; Chin-Dusting, J.; Remaley, A.T.; Sviridov, D. Structure/function relationships of apolipoprotein a-I mimetic peptides: Implications for antiatherogenic activities of high-density lipoprotein. Circ. Res. 2010, 107, 217–227. [Google Scholar] [CrossRef]
- Epand, R.M.; Shai, Y.; Segrest, J.P.; Anantharamaiah, G.M. Mechanisms for the modulation of membrane bilayer properties by amphipathic helical peptides. Biopolymers 1995, 37, 319–338. [Google Scholar] [CrossRef]
- Datta, G.; Epand, R.F.; Epand, R.M.; Chaddha, M.; Kirksey, M.A.; Garber, D.W.; Lund-Katz, S.; Phillips, M.C.; Hama, S.; Navab, M.; et al. Aromatic residue position on the nonpolar face of class a amphipathic helical peptides determines biological activity. J. Biol. Chem. 2004, 279, 26509–26517. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Van Lenten, B.J.; Navab, M.; Anantharamaiah, G.M.; Buga, G.M.; Reddy, S.T.; Fogelman, A.M. Multiple indications for anti-inflammatory apolipoprotein mimetic peptides. Curr. Opin. Investig. Drugs 2008, 9, 1157–1162. [Google Scholar]
- Epand, R.F.; Mishra, V.K.; Palgunachari, M.N.; Anantharamaiah, G.M.; Epand, R.M. Anti-inflammatory peptides grab on to the whiskers of atherogenic oxidized lipids. Biochim. Biophys. Acta 2009, 1788, 1967–1975. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.K.; Anantharamaiah, G.M.; Segrest, J.P.; Palgunachari, M.N.; Chaddha, M.; Sham, S.W.; Krishna, N.R. Association of a model class A (apolipoprotein) amphipathic alpha helical peptide with lipid: High resolution NMR studies of peptide.lipid discoidal complexes. J. Biol. Chem. 2006, 281, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Anantharamaiah, G.M.; Mishra, V.K.; Garber, D.W.; Datta, G.; Handattu, S.P.; Palgunachari, M.N.; Chaddha, M.; Navab, M.; Reddy, S.T.; Segrest, J.P.; et al. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J. Lipid Res. 2007, 48, 1915–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Hama, S.; Hough, G.; Grijalva, V.R.; Yu, N.; Ansell, B.J.; Datta, G.; Garber, D.W.; et al. Apolipoprotein A-I mimetic peptides. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Recio, C.; Maione, F.; Iqbal, A.J.; Mascolo, N.; De Feo, V. The Potential Therapeutic Application of Peptides and Peptidomimetics in Cardiovascular Disease. Front. Pharmacol. 2017, 7, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoekenbroek, R.M.; Stroes, E.S.; Hovingh, G.K. ApoA-I mimetics. In Handbook of Experimental Pharmacology; Barrett, J.E., Ed.; Springer Nature: Basel, Switzerland, 2015; Volume 224, pp. 631–648. [Google Scholar]
- White, C.R.; Garber, D.W.; Anantharamaiah, G.M. Anti-inflammatory and cholesterol-reducing properties of apolipoprotein mimetics: A review. J. Lipid Res. 2014, 55, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle Subclasses and Molecular Components. In High Density Lipoproteins: From Biological Understanding to Clinical Exploitation; von Eckardstein, A., Kardassis, D., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–51. [Google Scholar]
- Hafiane, A.; Genest, J. High density lipoproteins: Measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015, 3, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Messieres, M.; Ng, A.; Duarte, C.J.; Remaley, A.T.; Lee, J.C. Single-Particle Tracking of Human Lipoproteins. Anal. Chem. 2016, 88, 596–599. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; van Leent, M.M.T.; Lameijer, M.; Fisher, E.A.; Fayad, Z.A.; Pérez-Medina, C. High-Density Lipoprotein Nanobiologics for Precision Medicine. Acc. Chem. Res. 2018, 51, 127–137. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Cavigiolio, G.; Ishida, B.Y.; Zhang, S.; Kane, J.P.; Weisgraber, K.H.; Oda, M.N.; Rye, K.A.; Pownall, H.J.; et al. Morphology and structure of lipoproteins revealed by an optimized negative-staining protocol of electron microscopy. J. Lipid Res. 2011, 52, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Van Antwerpen, R.; Chen, G.C.; Pullinger, C.R.; Kane, J.P.; Labelle, M.; Krauss, R.M.; Lunachavez, C.; Forte, T.M.; Gilkey, J.C. Cryo-electron microscopy of low density lipoprotein and reconstituted discoidal high density lipoprotein: Imaging of the apolipoprotein moiety. J. Lipid Res. 1997, 38, 659–669. [Google Scholar] [PubMed]
- Dutta, M. Recent Advances in Single Particle Cryo-electron Microscopy and Cryo-electron Tomography to Determine the Structures of Biological Macromolecules. J. Indian Inst. Sci. 2018, 98, 231–245. [Google Scholar] [CrossRef]
- Wu, Z.; Gogonea, V.; Lee, X.; May, R.P.; Pipich, V.; Wagner, M.A.; Undurti, A.; Tallant, T.C.; Baleanu-Gogonea, C.; Charlton, F.; et al. The low resolution structure of ApoA1 in spherical high density lipoprotein revealed by small angle neutron scattering. J. Biol. Chem. 2011, 286, 12495–12508. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gogonea, V.; Lee, X.; Wagner, M.A.; Li, X.M.; Huang, Y.; Undurti, A.; May, R.P.; Haertlein, M.; Moulin, M.; et al. Double superhelix model of high density lipoprotein. J. Biol. Chem. 2009, 284, 36605–36619. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Calabresi, L.; Franceschini, G. Recombinant apolipoproteins for the treatment of vascular diseases. Atherosclerosis 1999, 142, 29–40. [Google Scholar] [CrossRef]
- Murphy, A.J.; Chin-Dusting, J.; Sviridov, D. Reconstituted HDL: A therapy for atherosclerosis and beyond. Clin. Lipid. 2009, 4, 731–739. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Dusting, G.J.; Cutri, B.; Bao, S.; Drummond, G.R.; Rye, K.A.; Barter, P.J. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 2005, 111, 1543–1550. [Google Scholar] [CrossRef]
- Kontush, A.; Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Dauteuille, C.; Baudouin, I.G.; Nègre-Salvayre, A.; Salvayre, R.; Calzada, C.; et al. Phosphatidylserine: A key player in multiple biological activities of HDL. Atherosclerosis 2014, 235, e32. [Google Scholar] [CrossRef]
- Darabi, M.; Kontush, A. Phosphatidylserine in atherosclerosis. Curr. Opin. Lipidol. 2016, 27, 414–420. [Google Scholar] [CrossRef]
- Besler, C.; Heinrich, K.; Riwanto, M.; Luscher, T.F.; Landmesser, U. High-density lipoprotein-mediated anti-atherosclerotic and endothelial-protective effects: A potential novel therapeutic target in cardiovascular disease. Curr. Pharm. Des. 2010, 16, 1480–1493. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Grégoire, J.; L’Allier, P.L.; Ibrahim, R.; Lespérance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.-A.; et al. Effects of Reconstituted High-Density Lipoprotein Infusions on Coronary AtherosclerosisA Randomized Controlled Trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Tricoci, P.; D’Andrea, D.M.; Gurbel, P.A.; Yao, Z.; Cuchel, M.; Winston, B.; Schott, R.; Weiss, R.; Blazing, M.A.; Cannon, L.; et al. Infusion of Reconstituted High-Density Lipoprotein, CSL112, in Patients With Atherosclerosis: Safety and Pharmacokinetic Results From a Phase 2a Randomized Clinical Trial. J. Am. Heart Assoc. 2015, 4, e002171. [Google Scholar] [CrossRef]
- Diditchenko, S.; Gille, A.; Pragst, I.; Stadler, D.; Waelchli, M.; Hamilton, R.; Leis, A.; Wright, S.D. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2202–2211. [Google Scholar] [CrossRef]
- Gille, A.; D’Andrea, D.; Tortorici, M.A.; Hartel, G.; Wright, S.D. CSL112 (Apolipoprotein A-I [Human]) Enhances Cholesterol Efflux Similarly in Healthy Individuals and Stable Atherosclerotic Disease Patients. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Nanjee, M.N.; Cooke, C.J.; Garvin, R.; Semeria, F.; Lewis, G.; Olszewski, W.L.; Miller, N.E. Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J. Lipid Res. 2001, 42, 1586–1593. [Google Scholar]
- Kootte, R.S.; Smits, L.P.; van der Valk, F.M.; Dasseux, J.L.; Keyserling, C.H.; Barbaras, R.; Paolini, J.F.; Santos, R.D.; van Dijk, T.H.; Dallinga-van Thie, G.M.; et al. Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J. Lipid Res. 2015, 56, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.H.; van der Valk, F.M.; Smits, L.P.; Sandberg, M.; Dasseux, J.L.; Baron, R.; Barbaras, R.; Keyserling, C.; Coolen, B.F.; Nederveen, A.J.; et al. HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis 2016, 251, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, S.J.; Andrews, J.; Kastelein, J.J.P.; Merkely, B.; Nissen, S.E.; Ray, K.K.; Schwartz, G.G.; Worthley, S.G.; Keyserling, C.; Dasseux, J.L.; et al. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 815–822. [Google Scholar]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef]
- Pownall, H.J.; Rosales, C.; Gillard, B.K.; Ferrari, M. Native and Reconstituted Plasma Lipoproteins in Nanomedicine: Physicochemical Determinants of Nanoparticle Structure, Stability, and Metabolism. Methodist DeBakey Cardiovasc. J. 2016, 12, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.K.; Lovell, J.F.; Zheng, G. Lipoprotein-Inspired Nanoparticles for Cancer Theranostics. Acc. Chem. Res. 2011, 44, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Everett, B.M. Novel Antiatherosclerotic Therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Molinaro, R.; Sellar, R.S.; Ebert, B.L. Jak-ing Up the Plaque’s Lipid Core…and Even More. Circ. Res. 2018, 123, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Lacko, A.G.; Sabnis, N.A.; Nagarajan, B.; McConathy, W.J. HDL as a drug and nucleic acid delivery vehicle. Front. Pharmacol. 2015, 6, 43. [Google Scholar] [CrossRef]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J.M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Song, Q.; Gao, X. Reconstituted high-density lipoproteins: Novel biomimetic nanocarriers for drug delivery. Acta Pharm. Sin. B 2018, 8, 51–63. [Google Scholar] [CrossRef]
- Raut, S.; Mooberry, L.; Sabnis, N.; Garud, A.; Dossou, A.S.; Lacko, A. Reconstituted HDL: Drug Delivery Platform for Overcoming Biological Barriers to Cancer Therapy. Front. Pharmacol. 2018, 9, 1154. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, W.; Jin, H.; Lovell, J.F.; Yang, M.; Ding, L.; Chen, J.; Corbin, I.; Luo, Q.; Zheng, G. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew. Chem. Int. Ed. 2009, 48, 9171–9175. [Google Scholar] [CrossRef]
- Muller, A.; Beck, K.; Rancic, Z.; Muller, C.; Fischer, C.R.; Betzel, T.; Kaufmann, P.A.; Schibli, R.; Kramer, S.D.; Ametamey, S.M. Imaging atherosclerotic plaque inflammation via folate receptor targeting using a novel 18F-folate radiotracer. Mol. Imaging 2014, 13, 1–11. [Google Scholar] [CrossRef]
- Corbin, I.R.; Chen, J.; Cao, W.; Li, H.; Lund-Katz, S.; Zheng, G. Enhanced cancer-targeted delivery using engineered high-density lipoprotein-based nanocarriers. J. Biomed. Nanotechnol. 2007, 3, 367–376. [Google Scholar] [CrossRef]
- Corbin, I.R.; Ng, K.K.; Ding, L.; Jurisicova, A.; Zheng, G. Near-infrared fluorescent imaging of metastatic ovarian cancer using folate receptor-targeted high-density lipoprotein nanocarriers. Nanomedicine (Lond.) 2013, 8, 875–890. [Google Scholar] [CrossRef] [Green Version]
- Mutharasan, R.K.; Foit, L.; Thaxton, C.S. High-Density Lipoproteins for Therapeutic Delivery Systems. J. Mater. Chem. B 2016, 4, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Shaish, A.; Keren, G.; Chouraqui, P.; Levkovitz, H.; Harats, D. Imaging of aortic atherosclerotic lesions by (125)I-LDL, (125)I-oxidized-LDL, (125)I-HDL and (125)I-BSA. Pathobiology 2001, 69, 225–229. [Google Scholar] [CrossRef]
- Cormode, D.P.; Briley-Saebo, K.C.; Mulder, W.J.; Aguinaldo, J.G.; Barazza, A.; Ma, Y.; Fisher, E.A.; Fayad, Z.A. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small 2008, 4, 1437–1444. [Google Scholar] [CrossRef]
- Cormode, D.P.; Chandrasekar, R.; Delshad, A.; Briley-Saebo, K.C.; Calcagno, C.; Barazza, A.; Mulder, W.J.; Fisher, E.A.; Fayad, Z.A. Comparison of synthetic high density lipoprotein (HDL) contrast agents for MR imaging of atherosclerosis. Bioconjugate Chem. 2009, 20, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Frias, J.C.; Ma, Y.; Chen, W.; Skajaa, T.; Briley-Saebo, K.; Barazza, A.; Williams, K.J.; Mulder, W.J.; Fayad, Z.A.; et al. HDL as a contrast agent for medical imaging. Clin. Lipidol. 2009, 4, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, A.C.; Choudhury, R.P. Form to function: Current and future roles for atherosclerosis imaging in drug development. Nat. Rev. Drug Discov. 2008, 7, 517–529. [Google Scholar] [CrossRef]
- Frias, J.C.; Williams, K.J.; Fisher, E.A.; Fayad, Z.A. Recombinant HDL-like nanoparticles: A specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 2004, 126, 16316–16317. [Google Scholar] [CrossRef]
- Sigalov, A.B. Nature-inspired nanoformulations for contrast-enhanced in vivo MR imaging of macrophages. Contrast Media Mol. Imaging 2014, 9, 372–382. [Google Scholar] [CrossRef]
- Frias, J.C.; Ma, Y.; Williams, K.J.; Fayad, Z.A.; Fisher, E.A. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006, 6, 2220–2224. [Google Scholar] [CrossRef]
- Briley-Saebo, K.C.; Geninatti-Crich, S.; Cormode, D.P.; Barazza, A.; Mulder, W.J.; Chen, W.; Giovenzana, G.B.; Fisher, E.A.; Aime, S.; Fayad, Z.A. High-relaxivity gadolinium-modified high-density lipoproteins as magnetic resonance imaging contrast agents. J. Phys. Chem. B 2009, 113, 6283–6289. [Google Scholar] [CrossRef]
- Briley-Saebo, K.C.; Mulder, W.J.; Mani, V.; Hyafil, F.; Amirbekian, V.; Aguinaldo, J.G.; Fisher, E.A.; Fayad, Z.A. Magnetic resonance imaging of vulnerable atherosclerotic plaques: Current imaging strategies and molecular imaging probes. J. Magn. Reson. Imaging 2007, 26, 460–479. [Google Scholar] [CrossRef]
- Agasti, S.S.; Rana, S.; Park, M.H.; Kim, C.K.; You, C.C.; Rotello, V.M. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010, 62, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Skajaa, T.; Cormode, D.P.; Falk, E.; Mulder, W.J.M.; Fisher, E.A.; Fayad, Z.A. High-Density Lipoprotein-Based Contrast Agents for Multimodal Imaging of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol 2010, 30, 169–176. [Google Scholar] [CrossRef]
- Cormode, D.P.; Skajaa, T.; van Schooneveld, M.M.; Koole, R.; Jarzyna, P.; Lobatto, M.E.; Calcagno, C.; Barazza, A.; Gordon, R.E.; Zanzonico, P.; et al. Nanocrystal Core High-Density Lipoproteins: A Multimodality Contrast Agent Platform. Nano Lett. 2008, 8, 3715–3723. [Google Scholar] [CrossRef] [Green Version]
- Skajaa, T.; Cormode, D.P.; Jarzyna, P.A.; Delshad, A.; Blachford, C.; Barazza, A.; Fisher, E.A.; Gordon, R.E.; Fayad, Z.A.; Mulder, W.J.M. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials 2011, 32, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Damiano, M.G.; Zhang, H.; Tripathy, S.; Luthi, A.J.; Rink, J.S.; Ugolkov, A.V.; Singh, A.T.; Dave, S.S.; Gordon, L.I.; et al. Biomimetic, synthetic HDL nanostructures for lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 2511–2516. [Google Scholar] [CrossRef] [Green Version]
- Luthi, A.J.; Lyssenko, N.N.; Quach, D.; McMahon, K.M.; Millar, J.S.; Vickers, K.C.; Rader, D.J.; Phillips, M.C.; Mirkin, C.A.; Thaxton, C.S. Robust passive and active efflux of cellular cholesterol to a designer functional mimic of high density lipoprotein. J. Lipid Res. 2015, 56, 972–985. [Google Scholar] [CrossRef] [Green Version]
- McMahon, K.M.; Mutharasan, R.K.; Tripathy, S.; Veliceasa, D.; Bobeica, M.; Shumaker, D.K.; Luthi, A.J.; Helfand, B.T.; Ardehali, H.; Mirkin, C.A.; et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011, 11, 1208–1214. [Google Scholar] [CrossRef]
- McMahon, K.M.; Thaxton, C.S. High-density lipoproteins for the systemic delivery of short interfering RNA. Expert Opin. Drug Deliv. 2014, 11, 231–247. [Google Scholar] [CrossRef]
- McMahon, K.M.; Plebanek, M.P.; Thaxton, C.S. Properties of Native High-Density Lipoproteins Inspire Synthesis of Actively Targeted In Vivo siRNA Delivery Vehicles. Adv. Funct. Mater. 2016, 26, 7824–7835. [Google Scholar] [CrossRef]
- Liu, X.; Suo, R.; Xiong, S.L.; Zhang, Q.H.; Yi, G.H. HDL drug carriers for targeted therapy. Clin. Chim. Acta 2013, 415, 94–100. [Google Scholar] [CrossRef]
- Raut, S.; Dasseux, J.L.; Sabnis, N.A.; Mooberry, L.; Lacko, A. Lipoproteins for therapeutic delivery: Recent advances and future opportunities. Ther. Deliv. 2018, 9, 257–268. [Google Scholar] [CrossRef]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014, 5, 3065. [Google Scholar] [CrossRef]
- Tang, J.; Lobatto, M.E.; Hassing, L.; van der Staay, S.; van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1, e1400223. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; He, H.; Liu, J.; Wang, J.; Zhang, S.; Zhang, S.; Wu, Z. Pharmacokinetics and atherosclerotic lesions targeting effects of tanshinone IIA discoidal and spherical biomimetic high density lipoproteins. Biomaterials 2013, 34, 306–319. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Xiao, Y.; Liu, J.-P.; Wu, Z.-M.; Gu, X.; Xu, Y.-M.; Lu, H. Structure and remodeling behavior of drug-loaded high density lipoproteins and their atherosclerotic plaque targeting mechanism in foam cell model. Int. J. Pharm. 2011, 419, 314–321. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lee, Y.; Boire, T.C.; Lee, J.-B.; Kim, W.S.; Sung, H.-J. Recent strategies to design vascular theranostic nanoparticles. Nanotheranostics 2017, 1, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Amar, M.J.; D’Souza, W.; Turner, S.; Demosky, S.; Sviridov, D.; Stonik, J.; Luchoomun, J.; Voogt, J.; Hellerstein, M.; Sviridov, D.; et al. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J. Pharmacol. Exp. Ther. 2010, 334, 634–641. [Google Scholar] [CrossRef]
- Watson, C.E.; Weissbach, N.; Kjems, L.; Ayalasomayajula, S.; Zhang, Y.; Chang, I.; Navab, M.; Hama, S.; Hough, G.; Reddy, S.T.; et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 2011, 52, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Leman, L.J.; Maryanoff, B.E.; Ghadiri, M.R. Molecules that mimic apolipoprotein A-I: Potential agents for treating atherosclerosis. J. Med. Chem. 2014, 57, 2169–2196. [Google Scholar] [CrossRef]
- Navab, M.; Reddy, S.; Van Lenten, B.J.; Anantharamaiah, G.M.; Fogelman, A.M. Role of dysfunctional HDL in atherosclerosis. J. Lipid Res. 2009, 50 (Suppl.), S145–S149. [Google Scholar] [CrossRef]
- Peterson, S.J.; Drummond, G.; Kim, D.H.; Li, M.; Kruger, A.L.; Ikehara, S.; Abraham, N.G. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J. Lipid Res. 2008, 49, 1658–1669. [Google Scholar] [CrossRef] [Green Version]
- Ruan, X.; Li, Z.; Zhang, Y.; Yang, L.; Pan, Y.; Wang, Z.; Feng, G.S.; Chen, Y. Apolipoprotein A-I possesses an anti-obesity effect associated with increase of energy expenditure and up-regulation of UCP1 in brown fat. J. Cell Mol. Med. 2011, 15, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Verghese, P.B.; Arrese, E.L.; Howard, A.D.; Soulages, J.L. Brefeldin A inhibits cholesterol efflux without affecting the rate of cellular uptake and re-secretion of apolipoprotein A-I in adipocytes. Arch. Biochem. Biophys. 2008, 478, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Van Lenten, B.J.; Wagner, A.C.; Jung, C.-L.; Ruchala, P.; Waring, A.J.; Lehrer, R.I.; Watson, A.D.; Hama, S.; Navab, M.; Anantharamaiah, G.M.; et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 2008, 49, 2302–2311. [Google Scholar] [CrossRef] [Green Version]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Buga, G.M.; Hough, G.; Wagner, A.C.; Fogelman, A.M. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: Novel hypotheses and review of literature. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2553–2560. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Navab, M.; Hough, G.; Gao, F.; Meriwether, D.; Grijalva, V.; Springstead, J.R.; Palgnachari, M.N.; Namiri-Kalantari, R.; Su, F.; et al. A novel approach to oral apoA-I mimetic therapy. J. Lipid Res. 2013, 54, 995–1010. [Google Scholar] [CrossRef] [Green Version]
- Navab, M.; Hough, G.; Buga, G.M.; Su, F.; Wagner, A.C.; Meriwether, D.; Chattopadhyay, A.; Gao, F.; Grijalva, V.; Danciger, J.S.; et al. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J. Lipid Res. 2013, 54, 3403–3418. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Hough, G.; Chattopadhyay, A.; Navab, M.; Fogelman, H.R.; Meriwether, D.; Williams, K.; Bensinger, S.; Moller, T.; Faull, K.F.; et al. Transgenic tomatoes expressing the 6F peptide and ezetimibe prevent diet-induced increases of IFN-beta and cholesterol 25-hydroxylase in jejunum. J. Lipid Res. 2017, 58, 1636–1647. [Google Scholar] [CrossRef]
- Navab, M.; Anantharamaiah, G.M.; Hama, S.; Hough, G.; Reddy, S.T.; Frank, J.S.; Garber, D.W.; Handattu, S.; Fogelman, A.M. D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1426–1432. [Google Scholar] [CrossRef]
- Bloedon, L.T.; Dunbar, R.; Duffy, D.; Pinell-Salles, P.; Norris, R.; DeGroot, B.J.; Movva, R.; Navab, M.; Fogelman, A.M.; Rader, D.J. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 2008, 49, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Dunbar, R.L.; Movva, R.; Bloedon, L.T.; Duffy, D.; Norris, R.B.; Navab, M.; Fogelman, A.M.; Rader, D.J. Oral Apolipoprotein A-I Mimetic D-4F Lowers HDL-Inflammatory Index in High-Risk Patients: A First-in-Human Multiple-Dose, Randomized Controlled Trial. Clin. Transl. Sci. 2017, 10, 455–469. [Google Scholar] [CrossRef]
- Sethi, A.A.; Stonik, J.A.; Thomas, F.; Demosky, S.J.; Amar, M.; Neufeld, E.; Brewer, H.B.; Davidson, W.S.; D’Souza, W.; Sviridov, D.; et al. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J. Biol. Chem. 2008, 283, 32273–32282. [Google Scholar] [CrossRef]
- Remaley, A.T.; Thomas, F.; Stonik, J.A.; Demosky, S.J.; Bark, S.E.; Neufeld, E.B.; Bocharov, A.V.; Vishnyakova, T.G.; Patterson, A.P.; Eggerman, T.L.; et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J. Lipid Res. 2003, 44, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Schwendeman, A.; Sviridov, D.O.; Yuan, W.; Guo, Y.; Morin, E.E.; Yuan, Y.; Stonik, J.; Freeman, L.; Ossoli, A.; Thacker, S.; et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 2015, 56, 1727–1737. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Black, A.S.; Bonnet, D.J.; Maryanoff, B.E.; Curtiss, L.K.; Leman, L.J.; Ghadiri, M.R. In vivo efficacy of HDL-like nanolipid particles containing multivalent peptide mimetics of apolipoprotein A-I. J. Lipid Res. 2014, 55, 2053–2063. [Google Scholar] [CrossRef] [Green Version]
- Navab, M.; Reddy, S.T.; Anantharamaiah, G.M.; Imaizumi, S.; Hough, G.; Hama, S.; Fogelman, A.M. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J. Lipid Res. 2011, 52, 1200–1210. [Google Scholar] [CrossRef] [Green Version]
- Di Bartolo, B.A.; Nicholls, S.J.; Bao, S.; Rye, K.-A.; Heather, A.K.; Barter, P.J.; Bursill, C. The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis 2011, 217, 395–400. [Google Scholar] [CrossRef]
- Di Bartolo, B.A.; Vanags, L.Z.; Tan, J.T.; Bao, S.; Rye, K.A.; Barter, P.J.; Bursill, C.A. The apolipoprotein A-I mimetic peptide, ETC-642, reduces chronic vascular inflammation in the rabbit. Lipids Health Dis. 2011, 10, 224. [Google Scholar] [CrossRef]
- Khan, M.; Lalwani, N.D.; Drake, S.L.; Crockatt, J.G.; Dasseux, J.L.H. Single-dose intravenous infusion of ETC-642, a 22-mer ApoA-I analogue and phospholipids complex, elevates HDL-C in atherosclerosis patients. Circulation 2003, 108, 563–564. [Google Scholar]
- Tanne, J.H. Pfizer stops clinical trials of heart drug. BMJ 2006, 333, 1237. [Google Scholar] [CrossRef]
- Chen, W.; Vucic, E.; Leupold, E.; Mulder, W.J.M.; Cormode, D.P.; Briley-Saebo, K.C.; Barazza, A.; Fisher, E.A.; Dathe, M.; Fayad, Z.A. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol. Imaging 2008, 3, 233–242. [Google Scholar] [CrossRef]
- Cormode, D.P.; Jarzyna, P.A.; Mulder, W.J.M.; Fayad, Z.A. Modified natural nanoparticles as contrast agents for medical imaging. Adv. Drug Deliv. Rev. 2010, 62, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.N.; Xu, L.; Han, Y.C.; Wang, Y.N.; Liu, G.; Qi, R. Recombinant high-density lipoproteins and their use in cardiovascular diseases. Drug Discov. Today 2017, 22, 180–185. [Google Scholar] [CrossRef]
- Zakiev, E.; Feng, M.; Sukhorukov, V.; Kontush, A. HDL-Targeting Therapeutics: Past, Present and Future. Curr. Pharm. Des. 2017, 23, 1207–1215. [Google Scholar] [CrossRef]
- Sviridov, D.; Remaley, A.T. High-density lipoprotein mimetics: Promises and challenges. Biochem. J. 2015, 472, 249–259. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Muhlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.C.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Hafner, A.; Lovric, J.; Lakos, G.P.; Pepic, I. Nanotherapeutics in the EU: An overview on current state and future directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar]







| Composition | Drug | Preclinical/Clinical | Activity/Primary Outcome | Ref. |
|---|---|---|---|---|
| rHDL Based Therapy in CVDs | ||||
| DPPC/purified human apo-AI | normocholesterolemic rabbit acute arterial inflammation model | neutrophil infiltration↓ ROS↓ adhesion molecules ↓ | [86] | |
| CSL-111: SoyPC/purified human apo AI (150/1 molar ratio) | patients with acute coronary syndromes | atheroma volume↓ | [90] | |
| CSL-112: SoyPC/purified human apo AI (75/1 molar ratio) | patients with stable atherothrombotic disease Phase 2a | apo-AI ↑ cholesterol efflux capacity↑ | [91] | |
| CER-001: recombinant apoAI/Egg SM/DPPG (1/2.7/0.1 molar ratios) | patients with carotid artery disease | apo-AI ↑ cholesterol efflux capacity↑ | [96] | |
| rHDL Drug Delivery | ||||
| Lyso-PC/DMPC/purified human apo-AI | simvastatin | apo E-/- mouse model | inflammation ↓ | [135] |
| SoyPC/cholesterol/purified apo-AI (disc) | tanshinone IIA | atherosclerotic NZW rabbit | cholesterol efflux capacity↑ | [136] |
| SoyPC/cholesterol/cholesteryl oleate/glycerol trioleate (spheres) | tanshinone IIA | atherosclerotic NZW rabbit | cholesterol efflux capacity↑ | [136] |
| rHDL apo-AI Mimetic Peptide | ||||
| POPC/5A (7/1 molar ratio) | Sprague-Dawley rats apo E-/- mouse model | cholesterol efflux capacity↑ atheroma area ↓ | [156] | |
| SM/5A (7/1 molar ratio) | Sprague-Dawley rats apo E-/- mouse model | cholesterol efflux capacity↑ atheroma area ↓ | [156] | |
| DMPC/branched, multivalent peptides | LDLr −/− mouse model | plasma total cholesterol levels ↓ | [157] | |
| ETC-642: 22A/SM/DPPC (1/1/1 molar ratios) | atherosclerotic NZW rabbit | ICAM ↓, VCAM ↓ vascular inflammation↓ oxLDL↓ | [159] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornmueller, K.; Vidakovic, I.; Prassl, R. Artificial High Density Lipoprotein Nanoparticles in Cardiovascular Research. Molecules 2019, 24, 2829. https://doi.org/10.3390/molecules24152829
Kornmueller K, Vidakovic I, Prassl R. Artificial High Density Lipoprotein Nanoparticles in Cardiovascular Research. Molecules. 2019; 24(15):2829. https://doi.org/10.3390/molecules24152829
Chicago/Turabian StyleKornmueller, Karin, Ivan Vidakovic, and Ruth Prassl. 2019. "Artificial High Density Lipoprotein Nanoparticles in Cardiovascular Research" Molecules 24, no. 15: 2829. https://doi.org/10.3390/molecules24152829
APA StyleKornmueller, K., Vidakovic, I., & Prassl, R. (2019). Artificial High Density Lipoprotein Nanoparticles in Cardiovascular Research. Molecules, 24(15), 2829. https://doi.org/10.3390/molecules24152829