Structural and Aggregation Features of a Human κ-Casein Fragment with Antitumor and Cell-Penetrating Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Expression and Purification of [U-13C,15N]-RL2 or [U-15N]-RL2
2.2. Labeling of [U-15N]-RL2 with a Paramagnetic MTSL Probe
2.3. Chromatographic Separation of the RL2 Dimers
2.4. EPR Measurements
2.5. NMR Sample Preparation, Data Collection and Processing
2.6. DLS Analysis
2.7. AFM
2.8. CD Spectroscopy
3. Results and Discussion
3.1. Study of RL2 Cytotoxic Activity
3.2. RL2 Aggregation Properties. DLS and AFM Analyses at Different pH Levels
3.3. EPR Analysis at Different pH Levels—Formation of Aggregates at Physiological pH
3.4. CD Analysis—The Secondary Structure of RL2 and Its Thermodynamic Stability
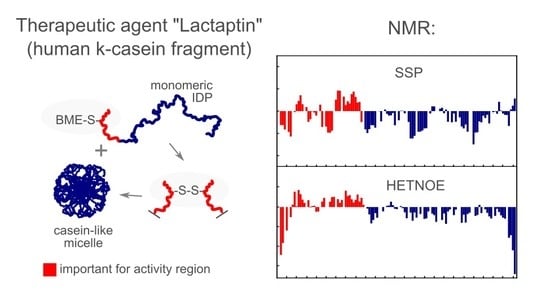
3.5. NMR Analysis. Dynamics and PRE Data on RL2 and MTSL-RL2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Floris, R.; Recio, I.; Berkhout, B.; Visser, S. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr. Pharm. Des. 2003, 9, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Kanyshkova, T.G.; Babina, S.E.; Semenov, D.V.; Isaeva, N.; Vlassov, A.V.; Neustroev, K.N.; Kul’minskaya, A.A.; Buneva, V.N.; Nevinsky, G.A. Multiple enzymic activities of human milk lactoferrin. Eur. J. Biochem. 2003, 270, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, M.; Tang, Y.; Wang, J.; Wei, C.; Gu, F.; Lei, T.; Chen, Z.; Qin, Y. The milk-derived fusion peptide, ACFP, suppresses the growth of primary human ovarian cancer cells by regulating apoptotic gene expression and signaling pathways. BMC Cancer 2016, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dou, Z.; Peng, X.; Hongjuan, W.; Shen, T.; Liu, J.; Li, G.; Gao, Y. Transcriptomics and proteomics analyses of anti-cancer mechanisms of TR35–An active fraction from Xinjiang Bactrian camel milk in esophageal carcinoma cell. Clin. Nutr. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Nemashkalova, E.L.; Kazakov, A.S.; Khasanova, L.M.; Permyakov, E.A.; Permyakov, S.E. Structural characterization of more potent alternatives to HAMLET, a tumoricidal complex of α-lactalbumin and oleic acid. Biochemistry 2013, 52, 6286–6299. [Google Scholar] [CrossRef] [PubMed]
- Richter, V.A.; Vaskova, A.A.; Koval, O.A.; Kuligina, E.V. Antitumor Potential of Lactaptin. Biol. Med. 2015, 2, 2. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; O’Keeffe, M.B.; FidzGerald, R.J. Milk Protein Hydrolysates and Bioactive Peptides. In Advanced Dairy Chemistry; McSweeney, P.H., O’Mahony, J.A., Eds.; Springer: New York, NY, USA, 2016; Volume 1B (15), pp. 417–482. [Google Scholar]
- Jøhnke, M.; Petersen, T.E. The Alpha-Lactalbumin/Oleic Acid Complex and Its Cytotoxic Activity. In Milk Ptotein; Hurley, W.L., Ed.; InTech: Rijeka, Croatia, 2012; Volume 1 (4), pp. 119–144. [Google Scholar]
- Nekipelaya, V.V.; Semenov, D.V.; Potapenko, M.O.; Kuligina, E.V.; Kit, Y.Y.; Romanova, I.V.; Richter, V.A. Lactaptin is a human milk protein inducing apoptosis of MCF-7 adenocarcinoma cells. Dokl. Biochem. Biophys. 2008, 419, 58–61. [Google Scholar] [CrossRef]
- Chinak, O.A.; Fomin, A.S.; Nushtaeva, A.A.; Koval, O.A.; Savelyeva, A.V.; Kuligina, E.V.; Richter, V.A. Penetration of the peptide lactaptin into human cancer cells. Russ. J. Bioorganic Chem. 2016, 42, 361–371. [Google Scholar] [CrossRef]
- Fomin, A.S.; Koval, O.A.; Semenov, D.V.; Potapenko, M.O.; Kuligina, E.V.; Kit, Y.Y.; Richter, V.A. Analysis of biochemical markers of MCF-7 cell apoptosis induced by a recombinant analogue of lactaptin. Russ. J. Bioorganic Chem. 2012, 38, 77–82. [Google Scholar] [CrossRef]
- Koval, O.A.; Tkachenko, A.V.; Fomin, A.S.; Semenov, D.V.; Nushtaeva, A.A.; Kuligina, E.V.; Zavjalov, E.L.; Richter, V.A. Lactaptin Induces p53-Independent Cell Death Associated with Features of Apoptosis and Autophagy and Delays Growth of Breast Cancer Cells in Mouse Xenografts. PLoS ONE 2014, 9, e93921. [Google Scholar] [CrossRef] [PubMed]
- Thorn, D.C.; Ecroyd, H.; Carver, J.A.; Holt, C. Casein structures in the context of unfolded proteins. Int. Dairy J. 2015, 46, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Kappa-casein, UniProtKB - P07498. Available online: https://www.uniprot.org/uniprot/P07498 (accessed on 28 May 2019).
- McGuffin, L.J. Intrinsic disorder prediction from the analysis of multiple protein fold recognition models. Bioinformatics 2008, 24, 1798–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, C.; Carver, J.A. Darwinian transformation of a ‘scarcely nutritious fluid’ into milk. J. Evol. Biol. 2012, 25, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, A.; Brookes, D.H.; Yost, S.R.; Dyson, H.J.; Forman-Kay, J.D.; Gunter, D.; Head-Gordon, M.; Hura, G.L.; Pande, V.S.; Wemmer, D.E.; et al. Finding Our Way in the Dark Proteome. J. Am. Chem. Soc. 2016, 138, 9730–9742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uversky, V.N.; Dunker, A.K. (Eds.) Intrinsically Disordered Protein Analysis; Springer: New York, NY, USA, 2012. [Google Scholar]
- Felli, I.C.; Pierattelli, R. (Eds.) Intrinsically Disordered Proteins Studied by NMR Spectroscopy; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Gibbs, E.B.; Cook, E.C.; Showalter, S.A. Application of NMR to studies of intrinsically disordered proteins. Arch. Biochem. Biophys. 2017, 628, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Kosol, S.; Contreras-Martos, S.; Cedeño, C.; Tompa, P. Structural Characterization of Intrinsically Disordered Proteins by NMR Spectroscopy. Molecules 2013, 18, 10802–10828. [Google Scholar] [CrossRef] [Green Version]
- Konrat, R. NMR contributions to structural dynamics studies of intrinsically disordered proteins. J. Magn. Reson. 2014, 241, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.G. NMR Characterization of the Dynamics of Biomacromolecules. Chem. Rev. 2004, 104, 3623–3640. [Google Scholar] [CrossRef]
- Showalter, S.A. Intrinsically Disordered Proteins: Methods for Structure and Dynamics Studies. In eMagRes; John Wiley & Sons, Ltd.: New York, NY, USA, 2014; pp. 181–190. [Google Scholar]
- Dyson, H.J.; Wright, P.E. Unfolded Proteins and Protein Folding Studied by NMR. Chem. Rev. 2004, 104, 3607–3622. [Google Scholar] [CrossRef]
- Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [PubMed]
- Vranken, W.F.; Boucher, W.; Stevens, T.J.; Fogh, R.H.; Pajon, A.; Llinas, M.; Ulrich, E.L.; Markley, J.L.; Ionides, J.; Laue, E.D. The CCPN data model for NMR spectroscopy: Development of a software pipeline. Proteins 2005, 59, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Semenov, D.V.; Fomin, A.S.; Kuligina, E.V.; Koval, O.A.; Matveeva, V.A.; Babkina, I.N.; Tikunova, N.V.; Richter, V.A. Recombinant Analogs of a Novel Milk Pro-Apoptotic Peptide, Lactaptin, and Their Effect on Cultured Human Cells. Protein J. 2010, 29, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Davis, B.G. Selective chemical protein modification. Nat. Commun. 2014, 5, 4740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalker, J.M.; Bernardes, G.J.L.; Lin, Y.A.; Davis, B.G. Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology. Chem. Asian J. 2009, 4, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Begg, G.E.; Speicher, D.W. Mass spectrometry detection and reduction of disulfide adducts between reducing agents and recombinant proteins with highly reactive cysteines. J. Biomol. Tech. JBT 1999, 10, 17–20. [Google Scholar] [PubMed]
- Packman, L.C.; Berry, A. A Reactive, Surface Cysteine Residue of the Class-II Fructose-1,6-Bisphosphate Aldolase of Escherichia coli Revealed by Electrospray Ionisation Mass Spectrometry. Eur. J. Biochem. 2019, 227, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Koval, O.A.; Fomin, A.S.; Kaledin, V.I.; Semenov, D.V.; Potapenko, M.O.; Kuligina, E.V.; Nikolin, V.P.; Nikitenko, E.V.; Richter, V.A. A novel pro-apoptotic effector lactaptin inhibits tumor growth in mice models. Biochimie 2012, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Aubry, S.; Burlina, F.; Dupont, E.; Delaroche, D.; Joliot, A.; Lavielle, S.; Chassaing, G.; Sagan, S. Cell-surface thiols affect cell entry of disulfide-conjugated peptides. FASEB J. 2009, 23, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Abegg, D.; Gasparini, G.; Hoch, D.G.; Shuster, A.; Bartolami, E.; Matile, S.; Adibekian, A. Strained Cyclic Disulfides Enable Cellular Uptake by Reacting with the Transferrin Receptor. J. Am. Chem. Soc. 2017, 139, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-L.; Oldfield, C.J.; Xue, B.; Meng, J.; Huang, F.; Romero, P.; Uversky, V.N.; Dunker, A.K. Exploring the binding diversity of intrinsically disordered proteins involved in one-to-many binding. Protein Sci. 2013, 22, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Thorn, D.C.; Meehan, S.; Sunde, M.; Rekas, A.; Gras, S.L.; MacPhee, C.E.; Dobson, C.M.; Wilson, M.R.; Carver, J.A. Amyloid Fibril Formation by Bovine Milk κ-Casein and Its Inhibition by the Molecular Chaperones αS- and β-Casein. Biochemistry 2005, 44, 17027–17036. [Google Scholar] [CrossRef] [PubMed]
- Raynes, J.; Day, L.; Crepin, P.; Horrocks, M.H.; Carver, J.A. Coaggregation of κ-Casein and β-Lactoglobulin Produces Morphologically Distinct Amyloid Fibrils. Small 2017, 13, 1603591. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Nørholm, A.B.; Hendus‒Altenburger, R.; Pedersen, S.F.; Poulsen, F.M.; Kragelund, B.B. Temperature-dependent structural changes in intrinsically disordered proteins: Formation of α‒helices or loss of polyproline II? Protein Sci. 2010, 19, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Natively unfolded proteins: A point where biology waits for physics. Protein Sci. 2002, 11, 739–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, C.; Herrada, I.; Celli, F.; Theillet, F.-X.; Zinn-Justin, S. 1H, 13C and 15N backbone resonance assignment of the intrinsically disordered region of the nuclear envelope protein emerin. Biomol. NMR Assign. 2016, 10, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Plowman, J.E.; Creamer, L.K.; Liddell, M.J.; Cross, J.J. Structural features of a peptide corresponding to human κ-casein residues 84–101 by 1H-nuclear magnetic resonance spectroscopy. J. Dairy Res. 1999, 66, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, T.; Dehle, F.C.; Musgrave, I.F.; Hoffmann, P.; Carver, J.A. Methionine Oxidation Enhances κ-Casein Amyloid Fibril Formation. J. Agric. Food Chem. 2012, 60, 4144–4155. [Google Scholar] [CrossRef] [PubMed]
- Salmon, L.; Nodet, G.; Ozenne, V.; Yin, G.; Jensen, M.R.; Zweckstetter, M.; Blackledge, M. NMR Characterization of Long-Range Order in Intrinsically Disordered Proteins. J. Am. Chem. Soc. 2010, 132, 8407–8418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, J.A.; Singh, V.K.; Jia, Z.; Forman-Kay, J.D. Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: Implications for fibrillation. Protein Sci. 2006, 15, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Bax, A. Prediction of Xaa-Pro peptide bond conformation from sequence and chemical shifts. J. Biomol. NMR 2009, 46, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abyzov, A.; Salvi, N.; Schneider, R.; Maurin, D.; Ruigrok, R.W.H.; Jensen, M.R.; Blackledge, M. Identification of Dynamic Modes in an Intrinsically Disordered Protein Using Temperature-Dependent NMR Relaxation. J. Am. Chem. Soc. 2016, 138, 6240–6251. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the recombinant analog RL2 is available from the authors. |








| pH 3.9 | pH 5.5 | pH 6 | pH 6.5 | pH 7 | pH 7.5 | pH 8 | ||
|---|---|---|---|---|---|---|---|---|
| No NaCl | Peak 1 | 11.7 ± 2.1 | 7.2 ± 0.7 | 6.9 ± 0.5 | - | 7.0 ± 0.9 | 7.0 ± 0.8 | - |
| Peak 2 | 288.4 ± 129.3 | 220.3 ± 60.8 | 210.5 ± 55.6 | 254.9 ± 58.6 | 147.8 ± 27.3 | 161.5 ± 39.4 | 170.7 ± 21.1 | |
| With NaCl | Peak 1 | 10.5 ± 1.2 | 6.6 ± 0.1 | 6.2 ± 0.3 | - | - | - | - |
| Peak 2 | 428.6 ± 167.8 | 196.1 ± 25.8 | 241.7 ± 40.8 | 255.9 ± 52.7 | 295.2 ± 18.1 | 699.4 ± 59.2 | 721.1 ± 35.0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinak, O.A.; Shernyukov, A.V.; Ovcherenko, S.S.; Sviridov, E.A.; Golyshev, V.M.; Fomin, A.S.; Pyshnaya, I.A.; Kuligina, E.V.; Richter, V.A.; Bagryanskaya, E.G. Structural and Aggregation Features of a Human κ-Casein Fragment with Antitumor and Cell-Penetrating Properties. Molecules 2019, 24, 2919. https://doi.org/10.3390/molecules24162919
Chinak OA, Shernyukov AV, Ovcherenko SS, Sviridov EA, Golyshev VM, Fomin AS, Pyshnaya IA, Kuligina EV, Richter VA, Bagryanskaya EG. Structural and Aggregation Features of a Human κ-Casein Fragment with Antitumor and Cell-Penetrating Properties. Molecules. 2019; 24(16):2919. https://doi.org/10.3390/molecules24162919
Chicago/Turabian StyleChinak, Olga A., Andrey V. Shernyukov, Sergey S. Ovcherenko, Evgeniy A. Sviridov, Victor M. Golyshev, Alexander S. Fomin, Inna A. Pyshnaya, Elena V. Kuligina, Vladimir A. Richter, and Elena G. Bagryanskaya. 2019. "Structural and Aggregation Features of a Human κ-Casein Fragment with Antitumor and Cell-Penetrating Properties" Molecules 24, no. 16: 2919. https://doi.org/10.3390/molecules24162919
APA StyleChinak, O. A., Shernyukov, A. V., Ovcherenko, S. S., Sviridov, E. A., Golyshev, V. M., Fomin, A. S., Pyshnaya, I. A., Kuligina, E. V., Richter, V. A., & Bagryanskaya, E. G. (2019). Structural and Aggregation Features of a Human κ-Casein Fragment with Antitumor and Cell-Penetrating Properties. Molecules, 24(16), 2919. https://doi.org/10.3390/molecules24162919