Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells
Abstract
:1. Introduction
2. Results
2.1. Structural Investigation of Liquid Crystalline Assemblies by Synchrotron BioSAXS
2.1.1. Design and Production of Self-Assembled Nanocarriers for the Loading of Catalase and Curcumin
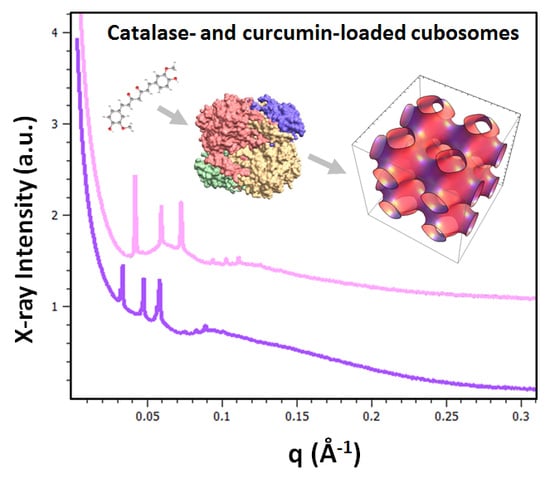
2.1.2. Liquid Crystalline Nanostructure Identification in MO/TPEG1000/FO/CU/CAT Systems by BioSAXS
2.1.3. Characterization of Liquid Crystalline Bulk Structures by BioSAXS
2.1.4. BioSAXS Characterization of Nanocarrier Dispersions
2.2. In Vitro Evaluation of Catalase- and Curcumin-Loaded Liquid Crystalline Nanocarriers
2.2.1. Viability of Cubosome Nanoparticle-Treated Differentiated SH-SY5Y Cells
2.2.2. Catalase Peroxidatic Activity in Cell Lysates of Differentiated SH-SY5Y Cells Obtained after Treatment with Cubosome Nanoparticles
3. Discussion
3.1. Structural Effect of Catalase Entrapped in Curcumin-Loaded Self-Assembled Liquid Crystalline Nanocarriers
3.2. Catalase Peroxidatic Function Following Cellular Treatment with Dual Drug-Loaded Cubosomes
4. Materials and Methods
4.1. Materials
4.2. Preparation of Bulk Liquid Crystalline MO/TPEG1000/Fish Oil/Curcumin Systems
4.3. Preparation of Aqueous Dispersions of Nanoparticles
4.4. Synchrotron Small Angle X-Ray Scattering (BioSAXS)
4.5. Nanoparticle Size Determination
4.6. Cell Culture
4.7. Cell Viability
4.8. Catalase Enzymatic Activity (Peroxidatic Function) in Supernatants of Cell Lysates
4.9. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkar, S.; Tran, N.; Rashid, M.H.; Le, T.C.; Yarovsky, I.; Conn, C.E.; Drummond, C.J. Toward cell membrane biomimetic lipidic cubic phases: A high-throughput exploration of lipid compositional space. ACS Appl. Bio Mater. 2019, 2, 182–195. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S. Biocompatible mesoporous and soft nanoarchitectures. J. Inorg. Organomet. Polym. 2015, 25, 214–232. [Google Scholar] [CrossRef]
- Larsson, K. Cubic lipid-water phases: Structures and biomembrane aspects. J. Phys. Chem. 1989, 93, 7304–7314. [Google Scholar] [CrossRef]
- Seddon, J.M.; Templer, R.H. Polymorphism of Lipid—Water Systems. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; Elsevier Science B.V.: North Holland, The Netherlands, 1995; Volume 1, pp. 99–149. [Google Scholar]
- Kulkarni, C.V. Lipid crystallization: From self-assembly to hierarchical and biological ordering. Nanoscale 2012, 4, 5779–5791. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Yaghmur, A.; Steinhart, M.; Kriechbaum, M.; Michael Rappolt, M. Effects of high pressure on internally self-assembled lipid nanoparticles: A synchrotron small-angle X-ray scattering (SAXS) study. Langmuir 2016, 32, 11907. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J. Surfactant systems: Their use in drug delivery. Chem. Soc. Rev. 1994, 23, 417–424. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc. Chem. Res. 2011, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Milak, S.; Zimmer, A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Stepanek, P.; Lesieur, S. Multicompartment lipid cubic nanoparticles with high protein upload: Millisecond dynamics of formation. ACS Nano 2014, 8, 5216–5226. [Google Scholar] [CrossRef]
- Garti, N.; Libster, D.; Aserin, A. Lipid polymorphism in lyotropic liquid crystals for triggered release of bioactives. Food Funct. 2012, 3, 700–713. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2018, 57, 2–23. [Google Scholar] [CrossRef]
- Han, S.; Shen, J.Q.; Gan, Y.; Geng, H.M.; Zhang, X.X.; Zhu, C.L.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef]
- Avachat, A.M.; Parpani, S.S. Formulation and development of bicontinuous nanostructured liquid crystalline particles of efavirenz. Colloids Surf. B: Biointerfaces 2015, 126, 87–97. [Google Scholar] [CrossRef]
- Aleandri, S.; Bandera, D.; Mezzenga, R.; Landau, E.M. Biotinylated cubosomes: A versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye. Langmuir 2015, 31, 12770–12776. [Google Scholar] [CrossRef]
- Baskaran, R.; Madheswaran, T.; Sundaramoorthy, P.; Kim, H.M.; Yoo, B.K. Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. Int. J. Nanomed. 2014, 9, 3119–3130. [Google Scholar] [Green Version]
- Fong, W.K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and fish oil-loaded spongosome and cubosome nanoparticles with neuroprotective potential against H2O2-induced oxidative stress in differentiated human SH-SY5Y cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Rades, T.; Boyd, B.; Boisen, A. Microcontainers as an oral delivery system for spray dried cubosomes containing ovalbumin. Eur. J. Pharm. Biopharm. 2017, 118, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, S.B.; Assmus, D.; Boehnke, A.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. Eur. J. Pharm. Biopharm. 2011, 79, 15–22. [Google Scholar] [CrossRef]
- Angelov, B.; Garamus, V.M.; Drechsler, M.; Angelova, A. Structural analysis of nanoparticulate carriers for encapsulation of macromolecular drugs. J. Mol. Liq. 2017, 235, 83–89. [Google Scholar] [CrossRef]
- Conn, C.E.; Drummond, C.J. Nanostructured bicontinuous cubic lipid self-assembly materials as matrices for protein encapsulation. Soft Matter 2013, 9, 3449. [Google Scholar] [CrossRef]
- Rizwan, S.B.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Liquid crystalline systems of phytantriol and glyceryl monooleate containing a hydrophilic protein: Characterisation, swelling and release kinetics. J. Pharm. Sci. 2009, 98, 4191–4204. [Google Scholar] [CrossRef]
- Angelova, A.; Ollivon, M.; Campitelli, A.; Bourgaux, C. Lipid cubic phases as stable nanochannel network structures for protein biochip development: X-ray diffraction study. Langmuir 2003, 19, 6928–6935. [Google Scholar] [CrossRef]
- Van’t Hag, L.; Shen, H.H.; Lin, T.W.; Gras, S.L.; Drummond, C.J.; Conn, C.E. Effect of lipid-based nanostructure on protein encapsulation within the membrane bilayer mimetic lipidic cubic phase using transmembrane and lipo-proteins from the beta-barrel assembly machinery. Langmuir 2016, 32, 12442–12452. [Google Scholar] [CrossRef]
- Van’t Hag, L.; De Campo, L.; Garvey, C.J.; Feast, G.C.; Leung, A.E.; Yepuri, N.R.; Knott, R.; Greaves, T.L.; Tran, N.; Gras, S.L.; et al. Using SANS with contrast-matched lipid bicontinuous cubic phases to determine the location of encapsulated peptides, proteins, and other biomolecules. J. Phys. Chem. Lett. 2016, 7, 2862–2866. [Google Scholar] [CrossRef]
- Darmanin, C.; Sarkar, S.; Castelli, L.; Conn, C.E. Effect of lipidic cubic phase structure on functionality of the dopamine 2l receptor: Implications for in meso crystallization. Cryst. Growth Des. 2016, 16, 5014–5022. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Garamus, V.M.; Lebas, G.; Lesieur, S.; Ollivon, M.; Funari, S.S.; Willumeit, R.; Couvreur, P. Small-angle neutron and X-ray scattering from amphiphilic stimuli-responsive diamond-type bicontinuous cubic phase. J. Am. Chem. Soc. 2007, 129, 13474–13479. [Google Scholar] [CrossRef]
- Valldeperas, M.; Wisniewska, M.; Ram-On, M.; Kesselman, E.; Danino, D.; Nylander, T.; Barauskas, J. Sponge phases and nanoparticle dispersions in aqueous mixtures of mono- and diglycerides. Langmuir 2016, 32, 8650–8659. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Garamus, V.M.; Drechsler, M. A vesicle-to-sponge transition via the proliferation of membrane-linking pores in w-3 polyunsaturated fatty acid-containing lipid assemblies. J. Mol. Liq. 2019, 279, 518–523. [Google Scholar] [CrossRef]
- Yaghmur, A.; Ghazal, A.; Ghazal, R.; Dimaki, M.; Svendsen, W.E. A hydrodynamic flow focusing microfluidic device for the continuous production of hexosomes based on docosahexaenoic acid monoglyceride. Phys. Chem. Chem. Phys. 2019, 21, 13005–13013. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Angelova, A.; Garamus, V.M.; Drechsler, M.; Willumeit, R.; Mutafchieva, R.; Štěpánek, P.; Lesieur, S. Earliest stage of the tetrahedral nanochannel formation in cubosome particles from unilamellar nanovesicles. Langmuir 2012, 28, 16647–16655. [Google Scholar] [CrossRef] [PubMed]
- Gontsarik, M.; Mohammadtaheri, M.; Yaghmur, A.; Salentinig, S. pH-Triggered nanostructural transformations in antimicrobial peptide/oleic acid self-assemblies. Biomater. Sci. 2018, 6, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, N.R.; Clulow, A.J.; Prentice, R.N.; Gilbert, E.P.; Hawley, A.; Rizwan, S.B.; Boyd, B.J.; Darwish, T.A. Deuterated phytantriol—A versatile compound for probing material distribution in liquid crystalline lipid phases using neutron scattering. J. Colloid Interface Sci. 2019, 534, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural Regen. Res. 2017, 12, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hu, J.; Huang, S.; Wang, B.; Siaw-Debrah, F.; Nyanzu, M.; Zhang, Y.; Zhuge, Q. Nanomaterial applications for neurological diseases and central nervous system injury. Prog. Neurobiol. 2017, 157, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid crystalline nanostructures as pegylated reservoirs of omega-3 polyunsaturated fatty acids: Structural insights toward delivery formulations against neurodegenerative disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef]
- Guerzoni, L.P.B.; Nicolas, V.; Angelova, A. In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharm. Res. 2017, 34, 492–505. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelova, A. Amphiphilic nanocarrier systems for curcumin delivery in neurodegenerative disorders. Medicines 2018, 5, 126. [Google Scholar] [CrossRef]
- Mueller, S.; Riedel, H.D.; Stremmel, W. Direct evidence for catalase as the predominant H2O2-removing enzyme in human erythrocytes. Blood 1997, 90, 4973–4978. [Google Scholar]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 26, 1095–1108. [Google Scholar] [CrossRef]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Giordano, C.R.; Roberts, R.; Krentz, K.A.; Bissig, D.; Talreja, D.; Kumar, A.; Terlecky, S.R.; Berkowitz, B.A. Catalase therapy corrects oxidative stress-induced pathophysiology in incipient diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2015, 56, 3095–3102. [Google Scholar] [CrossRef]
- Nishikawa, M.; Hashida, M.; Takakura, Y. Catalase delivery for inhibiting ROS- mediated tissue injury and tumor metastasis. Adv. Drug Deliv. Rev. 2009, 61, 319–326. [Google Scholar] [CrossRef]
- Vetrano, A.M.; Heck, D.E.; Mariano, T.M.; Mishin, V.; Laskin, D.L.; Laskin, J.D. Characterization of the oxidase activity in mammalian catalase. J. Biol. Chem. 2005, 280, 35372–35381. [Google Scholar] [CrossRef]
- Kremer, M.L. Peroxidatic activity of catalase. Biochim. Biophys. Acta 1970, 198, 199–209. [Google Scholar] [CrossRef]
- Laser, H. Peroxidatic activity of catalase. Biochem. J. 1955, 61, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Schriner, S.E.; Linford, N.J.; Martin, G.M.; Treuting, P.; Ogburn, C.E.; Emond, M.; Coskun, P.E.; Ladiges, W.; Wolf, N.; Van Remmen, H.; et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 2005, 308, 1909–1911. [Google Scholar] [CrossRef]
- Goth, L.; Rass, P.; Pay, A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. 2004, 8, 141–149. [Google Scholar] [CrossRef]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef]
- Kodydkova, J.; Vavrova, L.; Kocik, M.; Zak, A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol. 2014, 60, 153–167. [Google Scholar]
- Winternitz, M.C.; Meloy, C.R. On the occurrence of catalase in human tissues and its variations in diseases. J. Exp. Med. 1908, 10, 759–781. [Google Scholar] [CrossRef]
- Izawa, S.; Inoue, Y.; Kimura, A. Importance of catalase in the adaptive response to hydrogen peroxide: Analysis of acatalasaemic Saccharomyces cerevisiae. Biochem. J. 1996, 320, 61–67. [Google Scholar] [CrossRef]
- Rashtbari, S.; Dehghan, G.; Yekta, R.; Jouyban, A.; Iranshahi, M. Effects of resveratrol on the structure and catalytic function of bovine liver catalase (BLC): Spectroscopic and theoretical studies. Adv. Pharm. Bull. 2017, 7, 349–357. [Google Scholar] [CrossRef]
- Krych, J.; Gebicka, L. Catalase is inhibited by flavonoids. Int. J. Biol. Macromol. 2013, 58, 148–153. [Google Scholar] [CrossRef]
- Koohshekan, B.; Divsalar, A.; Saiedifar, M.; Saboury, A.A.; Ghalandari, B.; Gholamian, A.; Seyedarabi, A. Protective effects of aspirin on the function of bovine liver catalase: A spectroscopy and molecular docking study. J. Mol. Liq. 2016, 218, 8–15. [Google Scholar] [CrossRef]
- Najjar, M.F.; Ghadari, R.; Yousefi, R.; Safari, N.; Sheikhhasani, V.; Sheibani, N.; Moosavi-Movahedi, A.A. Studies to reveal the nature of interactions between catalase and curcumin using computational methods and optical techniques. Int. J. Biol. Macromol. 2017, 95, 550–556. [Google Scholar] [CrossRef]
- Najjar, M.F.; Taghavi, F.; Ghadari, R.; Sheibani, N.; Moosavi-Movahedi, A.A. Destructive effect of non-enzymatic glycation on catalase and remediation via curcumin. Arch. Biochem. Biophys. 2017, 630, 81–90. [Google Scholar] [CrossRef]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]
- Kozower, B.D.; Christopher-Solomidou, M.; Sweitzer, T.D.; Muro, S.; Buerk, D.G.; Solomides, C.C.; Albelda, S.M.; Patterson, G.A.; Muzykantov, V.R. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduce acute lung transplantation injury. Nat. Biotechnol. 2003, 21, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F.; Crapo, J.D.; Freeman, B.A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J. Clin. Invest. 1984, 73, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Morris, V.B.; Labhasetwar, V.; Ghorpade, A. Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress. Cell Death Dis. 2013, 4, e903. [Google Scholar] [CrossRef] [PubMed]
- Czechowska, E.; Ranoszek-Soliwoda, K.; Tomaszewska, E.; Pudlarz, A.; Celichowski, G.; Gralak-Zwolenik, D.; Szemraj, J.; Grobelny, J. Comparison of the antioxidant activity of catalase immobilized on gold nanoparticles via specific and non-specific adsorption. Colloids Surf. B: Biointerfaces 2018, 171, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; Fahmy, A.S.; Shaker, D.S.; Mohamed, S.A. Development of novel delivery system for nanoencapsulation of catalase: Formulation, characterization, and in vivo evaluation using oxidative skin injury model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H. Comprehensive studies on the nature of interaction between catalase and SiO2 nanoparticle. Mater. Res. Bull. 2014, 60, 51–56. [Google Scholar] [CrossRef]
- Bizien, T.; Durand, D.; Roblina, P.; Thureau, A.; Vachette, P.; Pérez, J. A brief survey of state-of-the-art BioSAXS. Protein Pept. Lett. 2016, 23, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Tuukkanen, A.T.; Spilotros, A.; Svergun, D.I. Progress in small-angle scattering from biological solutions at high-brilliance synchrotrons. IUCrJ 2017, 4, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Angelova, A.; Liu, J.; Garamus, V.M.; Angelov, B.; Zhang, X.; Li, Y.; Feger, G.; Li, N.; Zou, A. Self-assembly of mitochondria-specific peptide amphiphiles amplifying lung cancer cell death through targeting the VDAC1-hexokinase-II complex. J. Mater. Chem. B. 2019, 7, 4706–4716. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Matsumoto, K.; Lucarelli, E.; Thiele, C.J. Induction of Trkb by retinoic acid mediates biologic responsiveness to Bdnf and differentiation of human neuroblastoma cells. Neuron 1993, 11, 321–331. [Google Scholar] [CrossRef]
- Encinas, M.; Iglesias, M.; Liu, Y.H.; Wang, H.Y.; Muhaisen, A.; Cena, V.; Gallego, C.; Comella, J.X. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef]
- David, G.; Pérez, J. Combined sampler robot and high-performance liquid chromatography: A fully automated system for biological small-angle X-ray scattering experiments at the synchrotron SOLEIL SWING beamline. J. Appl. Crystallogr. 2009, 42, 892–900. [Google Scholar] [CrossRef]










| Samples | Liquid Crystalline Structures | Lattice a(Q) (nm) |
|---|---|---|
| C196 | Pn3m cubic | 18.5 |
| C197 | Pn3m cubic | 18.9 |
| C199 | Im3m cubic | 21.7 |
| C286 | Pn3m cubic | 20.0 |
| C287 | Cubic intermediate | - |
| C288 | Coexisting Pn3m cubic domains | 20.0/22.7 a |
| C376 | Cubic intermediate | - |
| C377 | Cubic intermediate | - |
| C378 | Pn3m cubic | 20.7 |
| C379 | Im3m cubic | 22.2 |
| C466 | Cubic intermediate | - |
| C467 | Cubic intermediate | - |
| C468 | Cubic intermediate—sponge | - |
| C469 | Pn3m cubic | 17.5 |
| Nanoparticles | Lattice a(Q) (nm) a | NPs’ Size (nm) b |
|---|---|---|
| Curcumin-Loaded NPs | ||
| MO-TPEG1000 | 1 | 106 |
| (MO-FO-CU)1 | 16.4/20.0 c | 106/220 d |
| (MO-FO-CU)2 | 20.0 | 220 |
| (MO-FO-CU)3 | 21.3 | 255 |
| Curcumin and Catalase-Loaded NPs | ||
| MO-CAT | - | 484 |
| (MO-FO-CU-CAT)1 | 23.2 | 164/550 b |
| (MO-FO-CU-CAT)2 | 25.0/26.3 a | 150/459 b |
| (MO-FO-CU-CAT) 3 | 26.3 | 164/531 b |
| Sample Code | Catalase (0.5 wt%) in Aqueous Buffer (pH 7) (g) | MO (g) | TPEG1000 (g) | FO (g) | CU (g) |
|---|---|---|---|---|---|
| Dilution Line (FO:MO) = DL (10:90) | |||||
| C195 | 0.013 | 0.0090 | 0.0020 | 0.0010 | 0.00013 |
| C196 | 0.015 | 0.0072 | 0.0018 | 0.0009 | 0.00010 |
| C197 | 0.018 | 0.0054 | 0.0013 | 0.0007 | 0.00008 |
| C199 | 0.023 | 0.0018 | 0.0004 | 0.0002 | 0.00003 |
| Dilution Line (FO:MO) = DL (20:80) | |||||
| C285 | 0.013 | 0.0080 | 0.0020 | 0.0022 | 0.00025 |
| C286 | 0.015 | 0.0066 | 0.0016 | 0.0016 | 0.00018 |
| C287 | 0.018 | 0.0050 | 0.0012 | 0.0011 | 0.00013 |
| C288 | 0.020 | 0.0032 | 0.0008 | 0.0009 | 0.00010 |
| Dilution Line (FO:MO) = DL (30:70) | |||||
| C376 | 0.015 | 0.0056 | 0.0014 | 0.0027 | 0.00030 |
| C377 | 0.018 | 0.0042 | 0.0010 | 0.0020 | 0.00023 |
| C378 | 0.020 | 0.0028 | 0.0007 | 0.0013 | 0.00015 |
| C379 | 0.023 | 0.0014 | 0.0003 | 0.0007 | 0.00008 |
| Dilution Line (FO:MO) = DL (40:60) | |||||
| C466 | 0.015 | 0.0048 | 0.0012 | 0.0036 | 0.00040 |
| C467 | 0.018 | 0.0036 | 0.0009 | 0.0027 | 0.00030 |
| C468 | 0.020 | 0.0025 | 0.0006 | 0.0017 | 0.00019 |
| C469 | 0.023 | 0.0012 | 0.0003 | 0.0009 | 0.00010 |
| 95 wt% Aqueous Phases | MO (g) | TPEG1000 (g) | FO (g) | CU (g) | |
|---|---|---|---|---|---|
| CU-Loaded NPs | CAT-Loaded NPs | ||||
| MO | MO-CAT | 0.02 | 0.005 | ||
| MO1-FO1 | 0.016 | 0.004 | 0.0045 | ||
| (MO-FO-CU)1 | (MO-FO-CU-CAT)1 | 0.016 | 0.004 | 0.0045 | 0.0005 |
| (MO-FO-CU)2 | (MO-FO-CU-CAT)2 | 0.012 | 0.003 | 0.0090 | 0.0010 |
| (MO-FO-CU)3 | (MO-FO-CU-CAT)3 | 0.008 | 0.002 | 0.0135 | 0.0015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. https://doi.org/10.3390/molecules24173058
Rakotoarisoa M, Angelov B, Espinoza S, Khakurel K, Bizien T, Angelova A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules. 2019; 24(17):3058. https://doi.org/10.3390/molecules24173058
Chicago/Turabian StyleRakotoarisoa, Miora, Borislav Angelov, Shirly Espinoza, Krishna Khakurel, Thomas Bizien, and Angelina Angelova. 2019. "Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells" Molecules 24, no. 17: 3058. https://doi.org/10.3390/molecules24173058
APA StyleRakotoarisoa, M., Angelov, B., Espinoza, S., Khakurel, K., Bizien, T., & Angelova, A. (2019). Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules, 24(17), 3058. https://doi.org/10.3390/molecules24173058