Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. C. lacinia Development
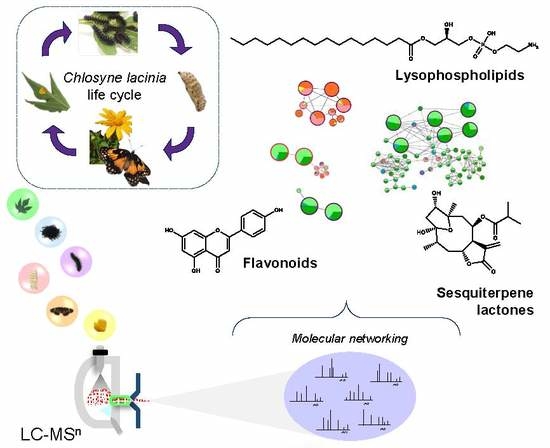
2.2. Natural Products Diversity
3. Material and Methods
3.1. Experimental Design
3.2. Sample Preparation
3.3. LC-MSn Analysis
3.4. Data Processing and Analysis
3.5. Chemical Analysis and Dereplication
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burkepile, D.E.; Parker, J.D. Recent advances in plant-herbivore interactions. F1000Research 2017, 6, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedio, B.E. Recent breakthroughs in metabolomics promise to reveal the cryptic chemical traits that mediate plant community composition, character evolution and lineage diversification. New Phytol. 2017, 214, 952–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, L.A.; Dyer, L.A.; Forister, M.L.; Smilanich, A.M.; Dodson, C.D.; Leonard, M.D.; Jeffrey, C.S. Phytochemical diversity drives plant–insect community diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 10973–10978. [Google Scholar] [CrossRef] [PubMed]
- Maag, D.; Erb, M.; Glauser, G. Metabolomics in plant-herbivore interactions: Challenges and applications. Entomol. Exp. Appl. 2015, 157, 18–29. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Rivas-Ubach, A. Ecological metabolomics: Overview of current developments and future challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Kuhlisch, C.; Pohnert, G. Metabolomics in chemical ecology. Nat. Prod. Rep. 2015, 32, 937–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, A.E.; Neto, F.C.; Vera, M.C.; Taboada, C.; Pavarini, D.P.; Bauermeister, A.; Lopes, N.P. An integrative omics perspective for the analysis of chemical signals in ecological interactions. Chem. Soc. Rev. 2018, 47, 1574–1591. [Google Scholar] [CrossRef]
- Zhou, S.; Lou, Y.-R.; Tzin, V.; Jander, G. Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef] [Green Version]
- Gobbo-Neto, L.; Bauermeister, A.; Sakamoto, H.; Gouvea, D.; Lopes, J.; Lopes, N. Spatial and temporal variations in secondary metabolites content of the Brazilian arnica leaves (Lychnophora ericoides Mart., Asteraceae). J. Braz. Chem. Soc. 2017, 28, 2382–2390. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Guaratini, T.; Pessoa, C.; De Moraes, M.O.; Costa-Lotufo, L.V.; Vieira, R.F.; Colepicolo, P.; Lopes, N.P. Differential metabolic and biological profiles of Lychnophora ericoides mart. (Asteraceae) from different localities in the Brazilian “campos rupestres”. J. Braz. Chem. Soc. 2010, 21, 750–759. [Google Scholar] [CrossRef]
- Barah, P.; Bones, A.M. Multidimensional approaches for studying plant defence against insects: From ecology to omics and synthetic biology. J. Exp. Bot. 2015, 66, 479–493. [Google Scholar] [CrossRef]
- Dyer, L.A.; Philbin, C.S.; Ochsenrider, K.M.; Richards, L.A.; Massad, T.J.; Smilanich, A.M.; Forister, M.L.; Parchman, T.L.; Galland, L.M.; Hurtado, P.J.; et al. Modern approaches to study plant–insect interactions in chemical ecology. Nat. Rev. Chem. 2018, 2, 50–64. [Google Scholar] [CrossRef]
- Baruah, N.C.; Sharma, R.P.; Madhusudanan, K.P.; Thyagarajan, G.; Herz, W.; Murari, R. Sesquiterpene lactones of Tithonia diversifolia. Stereochemistry of the tagitinins and related compounds. J. Org. Chem. 1979, 44, 1831–1835. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Xi, Z.-X.; Chen, W.-S.; Li, X.; Sun, L.; Sun, L.-N. Chemical constituents from Tithonia diversifolia and their chemotaxonomic significance. Biochem. Syst. Ecol. 2012, 44, 250–254. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Paula, D.A.; Oliveira, R.B.; Rocha, B.A.; Da Costa, F.B.; Chagas-Paula, D.A. Ethnobotany, chemistry, and biological activities of the genus Tithonia (Asteraceae). Chem. Biodivers. 2012, 9, 210–235. [Google Scholar] [CrossRef] [PubMed]
- Ajao, A.A.; Moteetee, A.N. Tithonia diversifolia (Hemsl) A. Gray. (Asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: A review. S. Afr. J. Bot. 2017, 113, 396–403. [Google Scholar] [CrossRef]
- Drummond, B.A., III; Bush, G.L.; Emmel, T.C. The biology and laboratory culture of Chlosyne lacinia Geyer (Nymphalidae). J. Lepid. Soc. 1970, 24, 135–142. [Google Scholar]
- Justus, C.M.; Pasini, A.; De Oliveira, É.D. Biologia e preferência da lagarta do girassol, Chlosyne lacinia saundersii (Lepidoptera: Nymphalidae) na planta daninha losna branca, Parthenium hysterophorus (Asteraceae). Neotrop. Entomol. 2003, 32, 163–166. [Google Scholar] [CrossRef]
- Neck, R.W. Foodplant ecology of the butterfly Chlosyne lacinia (Geyer) (Nymphalidae). I. Larval foodplants. J. Lepid. Soc. 1973, 27, 22–33. [Google Scholar]
- Lopes-Da-Silva, M.; Casagrande, M.M. Color polymorphism and allele frequency in a Brazilian population of the sunflower caterpillar Chlosyne lacinia saundersi (Doubleday) (Lepidoptera: Nymphalidae). Neotrop. Entomol. 2003, 32, 159–161. [Google Scholar] [CrossRef]
- Neck, R.W. Larval morph variation in Chlosyne lacinia (Nymphalidae). J. Lepid. Soc. 1976, 30, 91–94. [Google Scholar]
- Ambrósio, S.R.; Oki, Y.; Heleno, V.C.G.; Chaves, J.S.; Nascimento, P.G.B.D.; Lichston, J.E.; Constantino, M.G.; Varanda, E.M.; Da Costa, F.B. Constituents of glandular trichomes of Tithonia diversifolia: Relationships to herbivory and antifeedant activity. Phytochemistry 2008, 69, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, A.A.; Da Silva, R.; Knight, R.; Lopes, N.P.; Dorrestein, P.C. Global chemical analysis of biology by mass spectrometry. Nat. Rev. Chem. 2017, 1, 54. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beger, R.; Beale, M.H.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Fiehn, O. Strategies for dereplication of natural compounds using high-resolution tandem mass spectrometry. Phytochem. Lett. 2017, 21, 313–319. [Google Scholar] [CrossRef]
- Crotti, A.E.M.; Lopes, J.L.C.; Lopes, N.P. Triple quadrupole tandem mass spectrometry of sesquiterpene lactones: A study of goyazensolide and its congeners. J. Mass Spectrom. 2005, 40, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Herz, W. Biogenetic aspects of sesquiterpene lactone chemistry. Isr. J. Chem. 1977, 16, 32–44. [Google Scholar] [CrossRef]
- Dewick, P.M. The mevalonate and methylerythritol phosphate pathways: Terpenoids and steroids. In Medicinal Natural Products; Wiley: Hoboken, NJ, USA, 2009; pp. 187–310. [Google Scholar]
- Seaman, F.C. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot. Rev. 1982, 48, 121–594. [Google Scholar] [CrossRef]
- Emerenciano, V.D.P.; Ferreira, Z.S.; Kaplan, M.A.C.; Gottlieb, O.R. A chemosystematic analysis of tribes of Asteraceae involving sesquiterpene lactones and flavonoids. Phytochemistry 1987, 26, 3103–3115. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F. Systematics and evolution within the Compositae, seen with the eyes of a chemist. Plant Syst. Evol. 1990, 171, 1–14. [Google Scholar] [CrossRef]
- Da Costa, F.; Terfloth, L.; Gasteiger, J. Sesquiterpene lactone-based classification of three Asteraceae tribes: A study based on self-organizing neural networks applied to chemosystematics. Phytochemistry 2005, 66, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Gallon, M.E.; Monge, M.; Casoti, R.; Da Costa, F.B.; Semir, J.; Gobbo-Neto, L. Metabolomic analysis applied to chemosystematics and evolution of megadiverse Brazilian Vernonieae (Asteraceae). Phytochemistry 2018, 150, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Gonzalez, G.F.; Dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with High Toxicity. Crit. Rev. Plant Sci. 2016, 35, 1–20. [Google Scholar] [CrossRef]
- Martucci, M.E.P.; Gobbo-Neto, L. Differential secondary metabolite accumulation and performance of Chlosyne lacinia fed with Tithonia diversifolia or Vernonia polyanthes. Biochem. Syst. Ecol. 2016, 68, 156–162. [Google Scholar] [CrossRef]
- Ahern, J.R.; Whitney, K.D. Sesquiterpene lactone stereochemistry influences herbivore resistance and plant fitness in the field. Ann. Bot. 2014, 113, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.H.R.; Gil Da Costa, R.M.; Lopes, C.; Bastos, M.M.S.M. Sesquiterpene lactones: Adverse health effects and toxicity mechanisms. Crit. Rev. Toxicol. 2013, 43, 559–579. [Google Scholar] [CrossRef]
- Isman, M.B. Toxicity and tolerance of sesquiterpene lactones in the migratory grasshopper, Melanoplus sanguinipes (Acrididae). Pestic. Biochem. Physiol. 1985, 24, 348–354. [Google Scholar] [CrossRef]
- Dewick, P.M. The shikimate pathway: Aromatic amino acids and phenylpropanoids. In Medicinal Natural Products; Wiley: Hoboken, NJ, USA, 2009; pp. 137–186. [Google Scholar]
- Bone, K.; Mills, S. Principles of herbal pharmacology. In Principles and Practice of Phytotherapy: Modern Herbal Medicine; Churchill Livingstone: New York, NY, USA, 2013; p. 1056. ISBN 9780443069925. [Google Scholar]
- Silva, D.B.; Turatti, I.C.C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in Lychnophora species. Sci. Rep. 2014, 4, 4309. [Google Scholar] [CrossRef]
- Simmonds, M.S. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. J. Tradit. Complement. Med. 2017, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S. Flavonoid–insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Preiß, S.; Degenhardt, J.; Gershenzon, J. Plant-animal dialogues. In Ecological Biochemistry: Enviromental and Interspecies Interactions; Wiley-VCH: Weinheim, Germany, 2014; pp. 312–330. ISBN 9783527322909. [Google Scholar]
- Downer, R.G.H.; Matthews, J.R. Patterns of lipid distribution and utilization in insects. Am. Zool. 1976, 16, 733–745. [Google Scholar] [CrossRef]
- Fast, P.G. A comparative study of the phospholipids and fatty acids of some insects. Lipids 1966, 1, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.; Willett, D.S.; Harvey, J.A.; Alborn, H.T. Concurrence in the ability for lipid synthesis between life stages in insects. R. Soc. Open Sci. 2017, 4, 160815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Well, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.I. Lipid Metabolism and Function in Insects. Adv. Insect Physiol. 1967, 4, 69–211. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Riessberger-Gallé, U.; Hernández-López, J.; Rechberger, G.; Crailsheim, K.; Schuehly, W. Lysophosphatidylcholine acts in the constitutive immune defence against American foulbrood in adult honeybees. Sci. Rep. 2016, 6, 30699. [Google Scholar] [CrossRef] [Green Version]
- Pintea, A.M. Other natural pigments. In Food Colorants: Chemical and Functional Properties; Socaciu, C., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 101–124. [Google Scholar]
Sample Availability: Samples are not available from the authors. |





| Stage | 1st Instar | 2nd Instar | 3rd Instar | 4th Instar | 5th Instar | Pupation | Adult Stage | Total Development |
|---|---|---|---|---|---|---|---|---|
| Mean 1 | 5.25 | 3.70 | 3.25 | 3.45 | 3.55 | 7.65 | 11.35 | 38.20 |
| SD 2 | 1.12 | 0.65 | 0.44 | 0.83 | 0.69 | 0.67 | 1.04 | 0.85 |
| Rt | Usual Name (InChI) | Compound Class | Samples |
|---|---|---|---|
| 1.2 | pantothenic acid (1) (1/C9H17NO5/c1-9(2,5-11)7(14)8(15)10-4-3-6(12)13/h7,11,14H,3-5H2,1-2H3,(H,10,15)(H,12,13)/t7-/s2) | vitamin | T. diversifolia leaves and abaxial surface; C. lacinia feces, larvae, pupae, butterflies and eggs |
| 1.4 | tryptophan (2) (1/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/s2) | amino acid | T. diversifolia leaves and abaxial surface; C. lacinia larvae, pupae and butterflies |
| 3.2 | riboflavin (3) (1/C17H22N4O6/c1-7-3-9-10(4-8(7)2)21(5-11(23)14(25)12(24)6-22)15-13(18-9)16(26)20-17(27)19-15/h3-4,11-12,14-15,22-25H,5-6H2,1-2H3,(H2,19,20,26,27)/t11-,12+,14-,15?/s2) | vitamin | T. diversifolia leaves and abaxial surface; C. lacinia butterflies |
| 4.8 | phenylalanine-acetyl (4) (1/C11H13NO3/c1-8(13)12-10(11(14)15)7-9-5-3-2-4-6-9/h2-6,10H,7H2,1H3,(H,12,13)(H,14,15)/t10-/s2) | amino acid | T. diversifolia leaves; C. lacinia larvae, pupae and butterflies |
| 7.2 | isorhamnetin 3-O-hexoside (5) (1/C22H22O12/c1-31-12-4-8(2-3-10(12)25)20-21(17(28)15-11(26)5-9(24)6-13(15)32-20)34-22-19(30)18(29)16(27)14(7-23)33-22/h2-6,14,16,18-19,22-27,29-30H,7H2,1H3/t14-,16+,18+,19-,22+/s2) | flavonoid | T. diversifolia leaves and abaxial surface; C. lacinia feces and larvae |
| 7.6 | orizabin (6) (1/C19H26O7/c1-9(2)16(21)25-13-7-18(5)14(20)8-19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h6,9,12-15,20,23H,4,7-8H2,1-3,5H3/b10-6-/t12-,13-,14+,15+,18-,19-/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces and pupae |
| 7.7 | hispidulin 4′-O-hexoside (7) (1/C22H22O11/c1-30-21-12(25)7-14-16(18(21)27)11(24)6-13(32-14)9-2-4-10(5-3-9)31-22-20(29)19(28)17(26)15(8-23)33-22/h2-7,15,17,19-20,22-23,25-29H,8H2,1H3/t15-,17-,19+,20-,22-/s2) | flavonoid | C. lacinia feces and larvae |
| 8.7 | 1-hydroxy-3-O-methyltirotundin (8) (1/C20H30O7/c1-10(2)17(22)26-14-8-19(5)15(21)9-20(24-6,27-19)11(3)7-13-16(14)12(4)18(23)25-13/h10-11,13-16,21H,4,7-9H2,1-3,5-6H3/t11-,13+,14+,15-,16-,19+,20+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia butterflies |
| 9.4 | luteolin (9) (1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces |
| 9.6 | nepetin (10) (1S/C16H12O7/c1-22-16-11(20)6-13-14(15(16)21)10(19)5-12(23-13)7-2-3-8(17)9(18)4-7/h2-6,17-18,20-21H,1H3) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces |
| 9.9 | tagitinin A (11) (1/C19H28O7/c1-9(2)16(21)25-13-7-18(5)14(20)8-19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h9-10,12-15,20,23H,4,6-8H2,1-3,5H3/t10-,12+,13+,14-,15-,18+,19+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 10.3 | tagitinin B (12) (1/C19H26O7/c1-9(2)16(21)25-13-7-18(5)8-14(20)19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h6,9,12-15,20,23H,4,7-8H2,1-3,5H3/b10-6-/t12-,13-,14+,15+,18-,19+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 10.9 | apigenin (13) (1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces |
| 11.2 | hispidulin (14) (1S/C16H12O6/c1-21-16-11(19)7-13-14(15(16)20)10(18)6-12(22-13)8-2-4-9(17)5-3-8/h2-7,17,19-20H,1H3) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces and larvae |
| 11.4 | 2-hydroxytirotundin (15) (1/C19H28O7/c1-9(2)16(21)25-13-7-18(5)8-14(20)19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h9-10,12-15,20,23H,4,6-8H2,1-3,5H3/t10-,12+,13+,14+,15-,18+,19-/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces and butterflies |
| 11.7 | tagitinin C (16) (1/C19H24O6/c1-10(2)17(21)25-15-9-19(5,23)7-6-13(20)11(3)8-14-16(15)12(4)18(22)24-14/h6-8,10,14-16,23H,4,9H2,1-3,5H3/b7-6+,11-8-/t14-,15+,16-,19-/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 14.8 | 2-O-methyltagitinin B (17) (1/C20H28O7/c1-10(2)17(21)26-14-8-19(5)9-15(24-6)20(23,27-19)11(3)7-13-16(14)12(4)18(22)25-13/h7,10,13-16,23H,4,8-9H2,1-3,5-6H3/b11-7-/t13-,14-,15+,16+,19-,20+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 18.2 | 12,13-DiHOME (18) (1/C18H34O4/c1-2-3-10-13-16(19)17(20)14-11-8-6-4-5-7-9-12-15-18(21)22/h8,11,16-17,19-20H,2-7,9-10,12-15H2,1H3,(H,21,22)/b11-8-) | fatty acid derivative | T. diversifolia leaves and abaxial surface; C. lacinia feces, larvae, pupae, butterflies and eggs |
| 22.1 | 1-hexadecanoyl-glycero-3-phosphoethanolamine (19) (1/C21H44NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-21(24)27-18-20(23)19-29-30(25,26)28-17-16-22/h20,23H,2-19,22H2,1H3,(H,25,26)/t20-/s2) | lysoPL | T. diversifolia leaves and abaxial surface; C. lacinia pupae, butterflies and eggs |
| 22.5 | 1-palmitoyl-glycerol-3-phosphorylcholine (20) (1/C24H50NO7P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-24(27)30-21-23(26)22-32-33(28,29)31-20-19-25(2,3)4/h23,26H,5-22H2,1-4H3/p+1/t23-/s2) | lysoPL | T. diversifolia leaves; C. lacinia larvae, butterflies and eggs |
| 22.9 | 1-(9-octadecenoyl)-glycero-3-phosphoethanolamine (21) (1/C23H46NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-23(26)29-20-22(25)21-31-32(27,28)30-19-18-24/h9-10,22,25H,2-8,11-21,24H2,1H3,(H,27,28)/b10-9-/t22-/s2) | lysoPL | C. lacinia pupae, butterflies, eggs |
| 23.6 | 1-heptadecanoyl-glycero-3-phosphoethanolamine (22) (1/C22H46NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-22(25)28-19-21(24)20-30-31(26,27)29-18-17-23/h21,24H,2-20,23H2,1H3,(H,26,27)/t21-/s2) | lysoPL | T. diversifolia leaves; C. lacinia pupae and butterflies |
| 23.8 | 1-(9-octadecenoyl)-glycero-3-phosphoserine (23) (1/C24H46NO9P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-23(27)32-18-21(26)19-33-35(30,31)34-20-22(25)24(28)29/h9-10,21-22,26H,2-8,11-20,25H2,1H3,(H,28,29)(H,30,31)/b10-9-/t21-,22+/s2) | lysoPL | T. diversifolia leaves and abaxial surface; C. lacinia butterflies |
| 24.2 | 1-heptadecanoyl-glycero-3-phosphocholine (24) (1/C25H52NO7P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-25(28)31-22-24(27)23-33-34(29,30)32-21-20-26(2,3)4/h24,27H,5-23H2,1-4H3/p+1/t24-/s2) | lysoPL | T. diversifolia leaves and abaxial surface; C. lacinia pupae, butterflies and eggs |
| 24.4 | linolenoyl-tyrosine (25) (1/C27H39NO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(30)28-25(27(31)32)22-23-18-20-24(29)21-19-23/h3-4,6-7,9-10,18-21,25,29H,2,5,8,11-17,22H2,1H3,(H,28,30)(H,31,32)/b4-3-,7-6-,10-9-/t25-/s2) | fatty acid derivative | C. lacinia feces, larvae and pupae |
| 25.3 | 1-stearoyl-2-hydroxy-glycero-3-phosphoethanolamine (26) (1/C23H48NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-23(26)29-20-22(25)21-31-32(27,28)30-19-18-24/h22,25H,2-21,24H2,1H3,(H,27,28)/t22-/s2) | lysoPL | C. lacinia butterflies |
| 26.3 | phosphatidylethanolamine lyso alkenyl 18:0 (27) (1/C23H48NO6P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-19-28-23(21-25)22-30-31(26,27)29-20-18-24/h17,19,23,25H,2-16,18,20-22,24H2,1H3,(H,26,27)/b19-17+) | lysoPL | C. lacinia feces, larvae and butterflies |
| 29.3 | oleamide (28) (1S/C18H35NO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h9-10H,2-8,11-17H2,1H3,(H2,19,20)/b10-9-) | fatty acid derivative | T. diversifolia leaves and abaxial surface; C. lacinia butterflies and eggs |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallon, M.E.; Silva-Junior, E.A.; Amaral, J.G.; Lopes, N.P.; Gobbo-Neto, L. Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae). Molecules 2019, 24, 3118. https://doi.org/10.3390/molecules24173118
Gallon ME, Silva-Junior EA, Amaral JG, Lopes NP, Gobbo-Neto L. Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae). Molecules. 2019; 24(17):3118. https://doi.org/10.3390/molecules24173118
Chicago/Turabian StyleGallon, Marília Elias, Eduardo Afonso Silva-Junior, Juliano Geraldo Amaral, Norberto Peporine Lopes, and Leonardo Gobbo-Neto. 2019. "Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae)" Molecules 24, no. 17: 3118. https://doi.org/10.3390/molecules24173118
APA StyleGallon, M. E., Silva-Junior, E. A., Amaral, J. G., Lopes, N. P., & Gobbo-Neto, L. (2019). Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae). Molecules, 24(17), 3118. https://doi.org/10.3390/molecules24173118