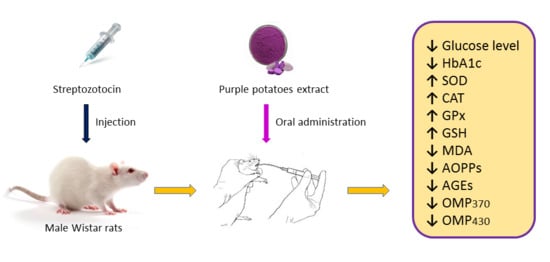
Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Content Determined by HPLC/LC–MS Method
2.2. Effect of Purple Potato (PP) Extract from Blue Congo Variety on Glucose-Related Parameters in STZ-Induced Diabetic Rats
2.3. The Effect of PP Extracts on Erythrocyte- and Leukocyte-Related Parameters
2.4. Measurement of Oxidative Stress-Related Enzymes in Leukocytes
2.5. The Effect of PP Extracts on Lipid Peroxidation in Blood Plasma of Rats
2.6. Effects of PP on the Content of Carbonyl-Oxidative Stress Metabolites in Rats′ Blood Plasma
2.7. Histopathology Study
2.7.1. Changes in Muscle Tissue
2.7.2. Changes in Kidney
2.7.3. Changes in Liver
3. Materials and Methods
3.1. Materials
3.2. Preparation of Extract from Blue Congo
3.3. Identification and Quantification of Compounds of Blue Congo Extract Using the UPLC–qTOF-MS/MS and HPLC–Photodiode Array (PDA) Methods
3.4. Experimental Animals
3.5. Induction of Diabetes Mellitus in Rats
3.6. Oral Glucose Tolerance Test
3.7. Collection of Blood and Preparation of Samples
3.8. Erythrocyte- and Leukocyte-Related Parameters
3.9. Assessment of Antioxidant Activities
3.9.1. Superoxide Dismutase
3.9.2. Catalase
3.9.3. Glutathione Peroxidase
3.9.4. Reduced Glutathione
3.10. Lipid Peroxidation
3.11. Assay of Advanced Oxidation Protein Products
3.12. Assay of Advanced Glycation End Products
3.13. Assay of Oxidative Modified Proteins
3.14. Histopathology Study
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lupascu, F.G.; Giusca, S.E.; Caruntu, I.D.; Anton, A.; Lupușoru, C.E.; Profire, L. The safety profile of new antidiabetic xanthine derivatives and their chitosan based formulations. Eur. J. Pharm. Sci. 2019, 127, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Shih, Z.; Kuo, Y.; Huang, G.; Tu, P.; Shih, C. Antidiabetic and antihyperlipidemic effects of the flower extract of Eriobotrya japonica in streptozotocin-induced diabetic mice and the potential bioactive constituents in vitro. J. Funct. Foods 2018, 49, 122–136. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Alsalmi, F.A. Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J. Biol. Sci. 2019, 26, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Landrault, N.; Poucheret, P.; Azay, J.; Krosniak, M.; Gasc, F.; Jenin, C.; Cros, G.; Teissedre, P.L. Effect of a polyphenols enriched chardonnay white wine in diabetic rats. J. Agric. Food Chem. 2003, 51, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Rommel, A.; Heatherbell, D.A.; Wrolstad, R.E. Red Raspberry Juice and Wine: Effect of Processing and Storage on Anthocyanin Pigment Composition, Color and Appearance. J. Food Sci. 1990, 55, 1011–1017. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Strugała, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef]
- Bontempo, P.; Carafa, V.; Grassi, R.; Basile, A.; Tenore, G.C.; Formisano, C.; Rigano, D.; Altucci, L. Antioxidant, antimicrobial and anti-proliferative activities of Solanum tuberosum L. var. Vitolette. Food Chem. Toxicol. 2013, 55, 304–312. [Google Scholar] [CrossRef]
- Luo, C.L.; Zhou, Q.; Yang, Z.W.; Wang, R.D.; Zhang, J.L. Evaluation of structure and bioprotective activity of key high molecular weight acylated anthocyanin compounds isolated from the purple sweet potato (Ipomoea batatas L. cultivar Eshu No.8). Food Chem. 2018, 241, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.; Davies, K.M.; Winefield, C. Anthocyanins: Biosynthesis, Functions and Applications; Springer: New York, NY, USA,, 2008. [Google Scholar]
- Oliveira, H.; Basílio, N.; Pina, F.; Fernandes, I.; de Freitas, V.; Mateus, N. Purple-fleshed sweet potato acylated anthocyanins: Equilibrium network and photophysical properties. Food Chem. 2019, 288, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.; Giusti, M. Anthocyanins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4398-9471-2. [Google Scholar]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.G.; Yan, Q.Q.; Lu, L.Z.; Zhang, Y.Q. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato. Nutr. Res. Pract. 2013, 7, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Yang, J.S.; Hwang, B.Y.; Yoo, B.K.; Han, K. Hypoglycemic effect of yacon tuber extract and its constituent, chlorogenic acid, in streptozotocin-induced diabetic rats. Biomol. Ther. 2009, 17, 256–262. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Okuno, S.; Yamaguchi, M.; Yamakawa, O. Antimutagenicity of deacylated anthocyanins in purple-fleshed sweet potato. Biosci. Biotechnol. Biochem. 2001, 65, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Lu, J.; Zheng, Y.; Li, J.; Zhou, Z.; Hu, B.; Zhang, Z.; Fan, S.; Mao, Z.; Wang, Y.-J.; et al. Purple sweet potato color ameliorates cognition deficits and attenuates oxidative damage and inflammation in aging mouse brain induced by D-galactose. J. Biomed. Biotechnol. 2009, 564737–564745. [Google Scholar] [CrossRef]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of Anthocyanin-enriched Purple-fleshed Sweet Potato P40 in Colorectal Cancer Prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Elsner, M.; Guldbakke, B.; Tiedge, M.; Munday, R.; Lenzen, S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetolgia 2007, 43, 1528–1533. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, V.P.; Gore, K.P.; Pawar, A.T. Antidiabetic, antihyperlipidemic activities and herb-drug interaction of a polyherbal formulation in streptozotocin induced diabetic rats. J. Ayurveda Integr. Med. 2017, 8, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 536–546. [Google Scholar]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Nandini, H.S.; Naik, P.R. Antidiabetic, antihyperlipidemic and antioxidant effect of Vincamine, in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2019, 843, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ieri, F.; Innocenti, M.; Andrenelli, L.; Vecchio, V.; Mulinacci, N. Rapid HPLC/DAD/MS method to determine phenolic acids, glycoalkaloids and anthocyanins in pigmented potatoes (Solanum tuberosum L.) and correlations with variety and geographical origin. Food Chem. 2011, 125, 750–759. [Google Scholar] [CrossRef]
- Alcade-Eon, C.; Saavedra, G.; de Pascual-Teresa, S.; Rivas Gonzalo, J.C. Identification of anthocyanins of pinta boca (Solanum stenotomum) tubers. Food Chem. 2004, 86, 441–448. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Hejtmánková, K.; Pazderů, K.; Dvořák, P.; Čepl, J. Impact of selected factors—Cultivar, storage, cooking and baking on the content of anthocyanins in coloured-flesh potatoes. Food Chem. 2012, 133, 1107–1116. [Google Scholar] [CrossRef]
- Nemś, A.; Pęksa, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kita, A.; Drożdż, W.; Hamouz, K. Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chem. 2015, 172, 175–182. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Bogucka, B. The influence of nitrogen and potassium fertilisation on the content of polyphenolic compounds and antioxidant capacity of coloured potato. J. Food Compost. Anal. 2016, 47, 69–75. [Google Scholar] [CrossRef]
- Hillebrand, S.; Naumann, H.; Kitzinski, N.; Kohler, N.; Winterhalter, P. Isolation and characterisation of anthocyanins from blue-fleshed potatoes (Solanum tuberosum L.). Food 2009, 3, 96–101. [Google Scholar]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- Sabahi, Z.; Khoshnood-Mansoorkhani, M.J.; Namadi, S.R.; Moein, M. Antidiabetic and Synergistic Effects of Anthocyanin Fraction from Berberis integerrima Fruit on Streptozotocin-Induced Diabetic Rats Model. Trends Pharmacol. Sci. 2016, 2, 43–50. [Google Scholar]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.H.; Kim, H.W.; Kim, S.Y.; Kim, S.M.; Kim, J.B.; Lee, Y.M. In vitro and in vivo hypoglycemic effects of cyanidin 3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside, an anthocyanin isolated from purple-fleshed sweet potato. Food Chem. 2019, 272, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Raffa, M.; Atig, F.; Mhalla, A.; Kerkeni, A.; Mechri, A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 2011, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Pan, Y.; Gao, X.; Chen, H. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food Funct. 2018, 9, 426–439. [Google Scholar] [CrossRef]
- Bila, I.; Dzydzan, O.; Brodyak, I.; Sybirna, N. Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus. Open Life Sci. 2019, 14, 299–310. [Google Scholar] [CrossRef]
- Subramani, S.; Leelavinothan, P. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem. Biol. Interact. 2012, 195, 43–51. [Google Scholar]
- Espinoza, S.E.; Guo, H.; Fedarko, N.; DeZern, A.; Fried, L.P.; Xue, Q.L.; Leng, S.; Beamer, B.; Walston, J.D. Glutathione Peroxidase Enzyme Activity in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 505–509. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburghb, K.H.; Lecourc, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, Y.; Sun, L.; Zhuang, Y. Anti-Diabetic Effects of Phenolic Extract from Rambutan Peels (Nephelium lappaceum) in High-Fat Diet and Streptozotocin-Induced Diabetic Mice. Nutrients 2017, 9, 801. [Google Scholar]
- Satriyasa, B.K. Aqueous extract of purple sweet potato tubers decrease MDA and increase SOD2 in kidney of diabetic rats. Bali Med. J. 2016, 5, 388–390. [Google Scholar] [CrossRef]
- Roy, M.; Sen, S.; Chakraborti, A.S. Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: Implication for glycation-induced hemoglobin modification. Life Sci. 2008, 23, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Kumar, D.; Abidi, A.B.; Rizvi, S.I. Efficacy of Composite Extract from Leaves and Fruits of Medicinal Plants Used in Traditional Diabetic Therapy against Oxidative Stress in Alloxan-Induced Diabetic Rats. ISRN Pharmacol. 2014, 2014, 4608590. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Zima, T.; Tesař, V.; Škrha, J.; Štípek, S. Determination of advanced glycation end-products and advanced oxidation protein products. Klin. Biochem. Metab. 2002, 10, 11–16. [Google Scholar]
- Almogbel, E.; Rasheed, N. Protein Mediated Oxidative Stress in Patients with Diabetes and its Associated Neuropathy: Correlation with Protein Carbonylation and Disease Activity Markers. J. Clin. Diagn. Res. 2017, F11, BC21–BC25. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin- induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef]
- Sasaki, R.; Nishimura, N.; Hoshino, H.; Isa, H.; Kadowaki, M.; Ichi, T.; Tanaka, A.; Nishiumi, S.; Fukuda, I.; Ashida, H.; et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007, 74, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. 2019, 13, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Widenmaier, S.B.; Schlein, C.; Johann, K.; Goncalves, R.L.S.; Eguchi, K.; Fischer, A.W.; Parlakgül, G.; Snyder, N.A.; Nguyen, T.B.; et al. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018, 24, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Mehenni, C.; Atmani-Kilani, D.; Dumarçay, S.; Perrin, D.; Gérardin, P.; Atmani, D. Hepatoprotective and antidiabetic effects of Pistacia lentiscus leaf and fruit extracts. J. Food Drug Anal. 2016, 24, 653–669. [Google Scholar] [CrossRef]
- Kjell, T.; Øyvind, M.A. Color stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Buko, V.; Zavodnik, I.; Kanuka, O.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Kirko, S.; Budryn, G.; Żyżelewicz, D.; Oracz, J.; et al. Antidiabetic effects and erythrocyte stabilization by red cabbage extract in streptozotocin-treated rats. Food Funct. 2018, 1, 1850–1863. [Google Scholar] [CrossRef]
- Vitak, Y.; Wasser, S.P.; Nevo, E.; Sybirna, N.O. The effect of the medicinal mushrooms Agaricus brasiliensis and Ganodermalucidum (higher basidiomycetes) on the erythron system in normal and streptozotocin-induced diabetic rats. Int. J. Med. Mushrooms 2015, 17, 277–286. [Google Scholar] [CrossRef]
- Souza, F.; Duncan, W.; Carvalho, R. Hematology and plasma biochemistry in rats fed with diets enriched with fatty fishes from Amazon region. Rev. Nutr. 2014, 27, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Melekh, B.; Ilkiv, I.; Lozynskyi, A.; Sklyarov, A. Antioxidant enzyme activity and lipid peroxidation in rat liver exposed to celecoxib and lansoprazole under epinephrine-induced stress. J. Appl. Pharm. Sci. 2017, 7, 94–99. [Google Scholar]
- Rizvi, S.I.; Zaid, M. A intracellular reduced glutathione content in normal and type 2 diabetic erythrocytes: Effect of insulin and (-)epicatechin. J. Physiol. Pharmacol. 2001, 52, 483–488. [Google Scholar]
- Putta, S.; Kilari, E.K. A review on methods of estimation of advanced glycation end products. World J. Pharm. Res. 2015, 4, 689–699. [Google Scholar]
- Demkovych, A.; Bondarenko, Y.; Hasiuk, P.A. Oxidative modification of proteins in the process of experimental periodontitis development. Interv. Med. Appl. Sci. 2017, 9, 218–221. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound Blue Congo extract are available from the authors. |








| Peak No. | Compound | Rt (min) | [M − H]+/[M + H]− (m/z) | MS/MS Fragments (m/z) | Content (mg/g d.m.) |
|---|---|---|---|---|---|
| Anthocyanins | |||||
| 1 | Petunidin-3-O-rutinoside-5-O-glucoside | 3.52 | 787.2288+ | 479.1120/317.0676 | 4.42 ± 0.23 |
| 2 | Petunidin-3-O-p-caffeyl-rutinoside-5-O-glucoside | 6.50 | 949.2610+ | 787.2059/479.1165/317.0676 | 7.41 ± 0.33 |
| 3 | Delphinidin-3-O-p-coumaroyl-rutinoside-5-O-glucoside | 6.68 | 919.2560+ | 757.2056/465.1026/303.0494 | 2.79 ± 0.24 |
| 4 | Petunidin-3-O-p-coumaryl-rutinoside-5-O-glucoside | 7.19 | 933.2700+ | 771.2118/479.1210/317.0676 | 53.95 ± 2.55 |
| 5 | Malwidin-3-O-p-coumaryl-rutinoside-5-O-glucoside | 7.69 | 947.2831+ | 785.2213/493.1294/131.0825 | 4.10 ± 0.19 |
| Phenolic Acid | |||||
| 1 | 3-O-Caffeoylquinic acid (neochlorogenic acid) | 3.38 | 353.0338− | 191.0562/179.0342 | 24.22 ± 1.03 |
| 2 | 4-O-Caffeoylquinic acid (cryptochlorogenic acid) | 4.59 | 353.0915− | 173.0458/179.0342/191.0534 | 29.69 ± 1.33 |
| 3 | Methyl-3-caffeoylquinate | 4.70 | 367.1057− | 161.0237 | 1.81 ± 0.11 |
| 4 | 5-O-Caffeoylquinic acid (chlorogenic acid) | 4.85 | 353.0838− | 707.1813/191.0563 | 98.65 ± 4.74 |
| 5 | Methyl-4-caffeoylquinate | 5.63 | 367.1051− | 161.0237 | 6.64 ± 0.26 |
| 6 | Methyl-5-caffeoylquinate | 6.41 | 367.1057− | 179.0342/161.0237/135.0450 | 6.39 ± 0.31 |
| Total | Anthocyanins and phenolic acid | 237.07 ± 11.32 | |||
| Parameters | Groups | |||
|---|---|---|---|---|
| C | C + PP | DM | DM + PP | |
| Number of leukocytes, 103 µL−1 | 10.14 ± 0.84 | 14.00 ± 0.63** | 11.17 ± 0.70 | 12.15 ± 1.11 |
| Number of red blood cell, 106 μL−1 | 5.48 ± 0.13 | 5.08 ± 0.23 | 6.06 ± 0.32 | 6.24 ± 0.41 |
| Hemoglobin content, g% | 15.00 ± 0.84 | 12.86 ± 0.56 | 15.75 ± 0.79 | 16.00 ± 0.44 |
| Mean cell hemoglobin, pg | 25.53 ± 0.77 | 25.50 ± 1.00 | 20.40 ± 1.67 | 25.58 ± 1.66# |
| Color index, c.u. | 0.77 ± 0.02 | 0.77 ± 0.03 | 0.62 ± 0.05 | 0.79 ± 0.05# |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strugała, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.Z.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michałowska, D.; Gabrielska, J.; et al. Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24, 3126. https://doi.org/10.3390/molecules24173126
Strugała P, Dzydzan O, Brodyak I, Kucharska AZ, Kuropka P, Liuta M, Kaleta-Kuratewicz K, Przewodowska A, Michałowska D, Gabrielska J, et al. Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats. Molecules. 2019; 24(17):3126. https://doi.org/10.3390/molecules24173126
Chicago/Turabian StyleStrugała, Paulina, Olha Dzydzan, Iryna Brodyak, Alicja Z. Kucharska, Piotr Kuropka, Mariana Liuta, Katarzyna Kaleta-Kuratewicz, Agnieszka Przewodowska, Dorota Michałowska, Janina Gabrielska, and et al. 2019. "Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats" Molecules 24, no. 17: 3126. https://doi.org/10.3390/molecules24173126
APA StyleStrugała, P., Dzydzan, O., Brodyak, I., Kucharska, A. Z., Kuropka, P., Liuta, M., Kaleta-Kuratewicz, K., Przewodowska, A., Michałowska, D., Gabrielska, J., & Sybirna, N. (2019). Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats. Molecules, 24(17), 3126. https://doi.org/10.3390/molecules24173126