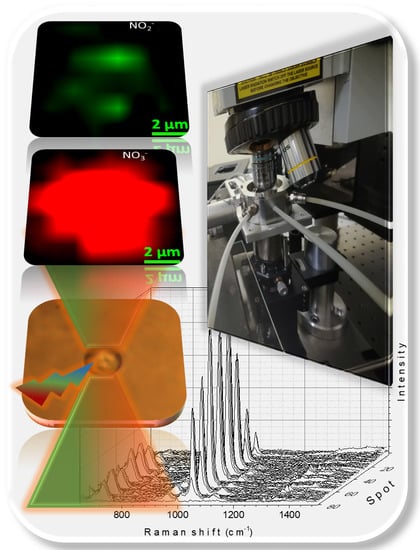
Enhancing Double-Beam Laser Tweezers Raman Spectroscopy (LTRS) for the Photochemical Study of Individual Airborne Microdroplets
Abstract
:1. Introduction
2. Instrumental Setup
2.1. Optical Tweezers
2.2. Confocal Raman Microspectrometer
2.3. Photochemical Levitation Chamber
2.4. Coupling of the Optical Tweezers with the Vertical Raman Microscope
3. Results and Discussion
4. Materials and Methods
4.1. Sample Preparation and Levitation
4.2. Photolysis of Levitated Particles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knox, K.J. Light-Induced Processes in Optically-Tweezed Aerosols Droplets; Springer: Berlin, Germany, 2011; p. 14. [Google Scholar]
- Krieger, U.K.; Marcolli, C.; Reid, J.P. Exploring the complexity of aerosol particle properties and processes using single particle techniques. Chem. Soc. Rev. 2012, 41, 6631. [Google Scholar] [CrossRef] [PubMed]
- Dennis-Smither, B.J.; Hanford, K.L.; Kwamena, N.A.; Miles, R.E.H.; Reid, J.P. Phase, morphology, and hygroscopicity of mixed oleic acid/sodium chloride/water aerosol particles before and after ozonolysis. J. Phys. Chem. A 2012, 116, 6159. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, S.; Suzuki, Y.; Kitamura, N. Laser trapping and picosecond time-resolved spectroscopy of water droplets in air: Cavity-enhanced spontaneous emission of Ru(bpy)3Cl2. Phys. Chem. Chem. Phys. 2010, 12, 9852. [Google Scholar] [CrossRef] [PubMed]
- Miles, R.E.H.; Walker, J.S.; Burnhamw, D.R.; Reid, J.P. Retrieval of the complex refractive index of aerosol droplets from optical tweezers measurements. Phys. Chem. Chem. Phys. 2012, 14, 3037. [Google Scholar] [CrossRef] [PubMed]
- Hunt, O.R.; Ward, A.D.; King, M.D. Heterogeneous oxidation of nitrite anion by gas-phase ozone in an aqueous droplet levitated by laser tweezers (optical trap): Is there any evidence for enhanced surface reaction? Phys. Chem. Chem. Phys. 2015, 17, 2734. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.F.; Wilson, K.R. Raman spectroscopy of isotopic water diffusion in ultraviscous, glassy, and gel states in aerosol by use of optical tweezers. Anal. Chem. 2016, 88, 2361. [Google Scholar] [CrossRef] [PubMed]
- Haddrell, A.E.; Miles, R.E.H.; Bzdek, B.R.; Reid, J.P.; Hopkins, R.J.; Walker, J.S. Coalescence sampling and analysis of aerosols using aerosol optical tweezers. Anal. Chem. 2017, 89, 2345. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, S.; Yamauchi, K.; Kitamura, N. In situ quantification of ammonium sulfate in single aerosol droplets by means of laser trapping and Raman spectroscopy. Anal. Sci. 2013, 29, 1223. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Hosny, N.A.; Tong, H.; Seville, P.C.; Gallimore, P.J.; Davidson, N.M.; Athanasiadis, A.; Botchway, S.W.; Ward, A.D.; Kalberer, M.; et al. Fluorescence lifetime imaging of optically levitated aerosol: A technique to quantitatively map the viscosity of suspended aerosol particles. Phys. Chem. Chem. Phys. 2016, 18, 21710–21719. [Google Scholar] [CrossRef]
- Meresman, H.; Hudson, A.J.; Reid, J.P. Spectroscopic characterization of aqueous microdroplets containing inorganic salts. Analyst 2011, 136, 3487. [Google Scholar] [CrossRef]
- Tobon, Y.A.; Seng, S.; Picone, L.A.; Bava, Y.B.; Juncal, L.C.; Moreau, M.; Romano, R.M.; Barbillat, J.; Sobanska, S. Photochemistry of single particles using acoustic levitation coupled with Raman microspectrometry. J. Raman Spectrosc. 2017, 48, 1135. [Google Scholar] [CrossRef]
- Seng, S.; Guo, F.; Tobón, Y.A.; Ishikawa, T.; Moreau, M.; Ishizaka, S.; Sobanska, S. Deliquescence behavior of photo-irradiated single NaNO3 droplets. Atmosph. Environ. 2018, 183, 33. [Google Scholar] [CrossRef]
- Thurn, R.; Kiefer, W. Raman-microsampling technique applying optical levitation by radiation pressure. Appl. Spectrosc. 1984, 38, 78. [Google Scholar] [CrossRef]
- Thurn, R.; Kiefer, W. Observations of structural resonances in the Raman spectra of optically levitated dielectric microspheres. J. Raman Spectrosc. 1984, 15, 411. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.; Videen, G.; Wang, C. Optical trapping and manipulation of single particles in air: Principles, technical details, and applications. J. Quant. Spectrosc. Radiat. Transf. 2018, 214, 94. [Google Scholar] [CrossRef]
- HongFei, M.; Yong, Z.; AnPei, Y. Single-cell discrimination based on optical tweezers Raman spectroscopy. Chin. Sci. Bull. 2013, 58, 2594. [Google Scholar] [CrossRef]
- Singh, G.P.; Creely, C.M.; Volpe, G.; Grotsch, H.; Petrov, D. Real-time detection of hyperosmotic stress response in optically trapped single yeast cells using Raman microspectroscopy. Anal. Chem. 2005, 77, 2564. [Google Scholar] [CrossRef]
- Casabella, S.; Scully, P.; Goddarda, N.; Gardner, P. Automated analysis of single cells using laser tweezers Raman spectroscopy. Analyst 2016, 141, 689. [Google Scholar] [CrossRef]
- Raj, S.; Marro, M.; Wojdyla, M.; Petrov, D. Mechanochemistry of single red blood cells monitored using Raman tweezers. Biomed. Opt. Express 2012, 3, 753. [Google Scholar] [CrossRef]
- Rao, S.; Bálint, S.; Cossins, B.; Guallar, V.; Petrov, D. Raman study of mechanically induced oxygenation state transition of red blood cells using optical tweezers. Biophys. J. 2009, 96, 209. [Google Scholar] [CrossRef]
- Huang, W.E.; Ward, A.D.; Whiteley, A.S. Raman tweezers sorting of single microbial cells. Environ. Microbiol. Rep. 2009, 1, 44. [Google Scholar] [CrossRef]
- Bankapur, A.; Zachariah, E.; Chidangil, S.; Valiathan, M.; Mathur, D. Raman tweezers spectroscopy of live, single red and white blood cells. PLoS ONE 2010, 5, e10427. [Google Scholar] [CrossRef]
- Xie, C.; Mace, J.; Dinno, M.A.; Li, Y.Q.; Tang, W.; Newton, R.J.; Gemperline, P.J. Identification of single bacterial cells in aqueous solution using confocal laser tweezers Raman spectroscopy. Anal. Chem. 2005, 77, 4390. [Google Scholar] [CrossRef]
- Xie, C.; Nguyen, N.; Zhu, Y.; Li, Y. Detection of the recombinant proteins in single transgenic microbial cell using laser tweezers and Raman spectroscopy. Anal. Chem. 2007, 79, 9269. [Google Scholar] [CrossRef]
- Chen, D.; Shelenkova, L.; Li, Y.; Kempf, C.R.; Sabelnikov, A. Laser tweezers Raman spectroscopy potential for studies of complex dynamic cellular processes: Single cell bacterial lysis. Anal. Chem. 2009, 81, 3227. [Google Scholar] [CrossRef]
- Moritz, T.J.; Taylor, D.S.; Polage, C.R.; Krol, D.M.; Lane, S.M.; Chan, J.W. Effect of cefazolin treatment on the nonresonant Raman signatures of the metabolic state of individual Escherichia coli cells. Anal. Chem. 2010, 82, 2703. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, P.; Setlow, P.; Li, Y. Characterization of bacterial spore germination using integrated phase contrast microscopy, Raman spectroscopy, and optical tweezers. Anal. Chem. 2010, 82, 3840. [Google Scholar] [CrossRef]
- Avetisyan, A.; Jensen, J.B.; Huser, T. Monitoring trehalose uptake and conversion by single bacteria using laser tweezers Raman spectroscopy. Anal. Chem. 2013, 85, 7264. [Google Scholar] [CrossRef]
- Rusciano, G.; de Luca, A.C.; Sasso, A. Enhancing Raman tweezers by phase-sensitive detection. Anal. Chem. 2007, 79, 3708. [Google Scholar] [CrossRef]
- Shoji, T.; Nohara, R.; Kitamura, N.; Tsuboi, Y. A method for an approximate determination of a polymer-rich-domain concentration in phase-separated poly(N-isopropylacrylamide) aqueous solution by means of confocal Raman microspectroscopy combined with optical tweezers. Anal. Chim. Acta 2015, 854, 118. [Google Scholar] [CrossRef]
- Creely, C.M.; Singh, G.P.; Petrov, D. Dual wavelength optical tweezers for confocal Raman spectroscopy. Opt. Commun. 2005, 245, 465. [Google Scholar] [CrossRef]
- Vasi, S.; Monaca, M.A.; Donato, M.G.; Bonaccorso, F.; Privitera, G.; Trushkevych, O.; Calogero, G.; Fazio, B.; Irrera, A.; Iatí, M.A.; et al. Optical trapping of carbon nanotubes and graphene. Atti Accad. Pelorit. Pericol. 2011, 89. [Google Scholar] [CrossRef]
- Shoji, T.; Kitamura, N.; Tsuboi, Y. Resonant excitation effect on optical trapping of myoglobin: The important role of a heme cofactor. J. Phys. Chem. C. 2013, 117, 10691. [Google Scholar] [CrossRef]
- Geβner, R.; Winter, C.; Rösch, P.; Schmitt, M.; Petry, R.; Kierfer, W.; Lankers, M.; Popp, J. Identification of biotic and abiotic particles by using a combination of optical tweezers and in situ Raman spectroscopy. Chem. Phys. Chem. 2004, 5, 1159. [Google Scholar] [CrossRef]
- Mao, H.; Luchette, P. An integrated laser-tweezers instrument for microanalysis of individual protein aggregates. Sens. Actuator B 2008, 129, 764. [Google Scholar] [CrossRef]
- Ramser, K.; Enger, J.; Goksör, M.; Hanstorp, D.; Logg, K.; Käll, M. A microfluidic system enabling Raman measurements of the oxygenation cycle in single optically trapped red blood cells. Lab. Chip. 2005, 5, 431. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.; Videen, G.; Wang, C. Optical trapping-Raman spectroscopy (OT-RS) with embedded microscopy imaging for concurrent characterization and monitoring of physical and chemical properties of single particles. Anal. Chim. Acta 2018, 86. [Google Scholar] [CrossRef]
- Ashok, P.C.; Dholakia, K. Optical trapping for analytical biotechnology. Curr. Opin. Biotechnol. 2012, 23, 16. [Google Scholar] [CrossRef]
- Dochow, S.; Krafft, C.; Neugebauer, U.; Bocklitz, T.; Henkel, T.; Mayer, G.; Albert, J.; Popp, J. Tumour cell identification by means of Raman spectroscopy in combination with optical traps and microfluidic environments. Lab. Chip. 2011, 11, 1484. [Google Scholar] [CrossRef]
- Kalume, A.; Zhu, E.; Wang, C.; Santarpia, J.; Pan, Y. Position-resolved Raman spectra from a laser-trapped single airborne chemical droplet. Opt. Lett. 2017, 42, 5113. [Google Scholar] [CrossRef]
- Yu, J.-Y.; Zhang, Y.; Tan, S.-H.; Liu, Y.; Zhang, Y.-H. Observation on the ion association equilibria in NaNO3 droplets using micro-Raman spectroscopy. J. Phys. Chem. B 2012, 116, 12581. [Google Scholar] [CrossRef]
- Belyi, M.U.; Gaididei, G.I.; Sakun, V.P.; Skryshevskaya, M.G. Concentration effects in the vibrational spectra of aqueous solutions of nitrites. J. Appl. Spectrosc. 1995, 62, 76. [Google Scholar] [CrossRef]
- Tsai, J.-H.M.; Harrison, J.G.; Martin, J.C.; Hamilton, T.P.; van derWoerd, M.; Jablonsky, M.J.; Beckman, J.S. Role of conformation of peroxynitrite anion (ONOO−) with its stability and toxicity. J. Am. Chem. Soc. 1994, 116, 4115. [Google Scholar] [CrossRef]
- Goldstein, S.; Rabani, J. Mechanism of nitrite formation by nitrate photolysis in aqueous solutions: the role of peroxynitrite, nitrogen dioxide, and hydroxyl radical. J. Am. Chem. Soc. 2007, 129, 10597. [Google Scholar] [CrossRef]
- Mark, G.; Korth, H.-G.; Schuchmann, H.-P.; von Sonntag, C. The photochemistry of aqueous nitrate ion revisited. J. Photochem. Photobiol. A 1996, 101, 89. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A 1999, 128, 1–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound sodium nitrate is available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Castaño, J.A.; Boussekey, L.; Verwaerde, J.P.; Moreau, M.; Tobón, Y.A. Enhancing Double-Beam Laser Tweezers Raman Spectroscopy (LTRS) for the Photochemical Study of Individual Airborne Microdroplets. Molecules 2019, 24, 3325. https://doi.org/10.3390/molecules24183325
Gómez Castaño JA, Boussekey L, Verwaerde JP, Moreau M, Tobón YA. Enhancing Double-Beam Laser Tweezers Raman Spectroscopy (LTRS) for the Photochemical Study of Individual Airborne Microdroplets. Molecules. 2019; 24(18):3325. https://doi.org/10.3390/molecules24183325
Chicago/Turabian StyleGómez Castaño, Jovanny A., Luc Boussekey, Jean P. Verwaerde, Myriam Moreau, and Yeny A. Tobón. 2019. "Enhancing Double-Beam Laser Tweezers Raman Spectroscopy (LTRS) for the Photochemical Study of Individual Airborne Microdroplets" Molecules 24, no. 18: 3325. https://doi.org/10.3390/molecules24183325
APA StyleGómez Castaño, J. A., Boussekey, L., Verwaerde, J. P., Moreau, M., & Tobón, Y. A. (2019). Enhancing Double-Beam Laser Tweezers Raman Spectroscopy (LTRS) for the Photochemical Study of Individual Airborne Microdroplets. Molecules, 24(18), 3325. https://doi.org/10.3390/molecules24183325