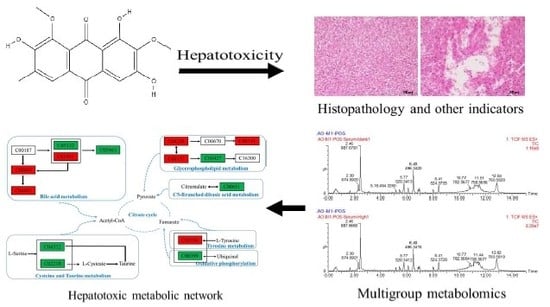
Metabolomics of Aurantio-Obtusin-Induced Hepatotoxicity in Rats for Discovery of Potential Biomarkers
Abstract
:1. Introduction
2. Results
2.1. Body Weights and Organ Coefficient
2.2. Toxicological Examination
2.3. Histopathological Examination of Liver
2.4. Analysis of Serum Metabolites in Sprague–Dawley Rats with Aurantio-Obtusin-Induced Hepatotoxicity
2.4.1. Methodology Validation
2.4.2. Metabolic Spectrum of Rat Serum
2.4.3. Metabolic Track Analysis of Rat Serum
2.4.4. Identification of Biomarkers
2.4.5. Metabolic Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Materials
4.2. Animal Study Design
4.3. Sample Collection and Preparation
4.4. Sample Analysis
4.4.1. Hematology, Serum Biochemistry, and Histopathological Analysis
4.4.2. Preparation of Quality Control Samples
4.4.3. Metabolomics Analyses
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Li, F.; Li, Y.; Li, W.; Xu, J.; Du, H. A review of traditional and current methods used to potentially reduce toxicity of Aconitum roots in Traditional Chinese Medicine. J. Ethnopharmacol. 2017, 207, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yan, G.L.; Han, Y.; Sun, H.; Zhang, A.H.; Li, X.N.; Wang, X.J. UPLC-Q-TOF/MS-based metabolomic studies on the toxicity mechanisms of traditional Chinese medicine Chuanwu and the detoxification mechanisms of Gancao, Baishao, and Ganjiang. Chin. J. Nat. Med. 2015, 13, 687–698. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, M.; Fu, H.B.; Xiao, H.T.; Bian, Z.X. Pre-clinical toxicity of a combination of berberine and 5-aminosalicylic acid in mice. Food Chem. Toxicol. 2016, 97, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kheir, M.M.; Wang, Y.; Hua, L.; Hu, J.; Li, L.; Lei, F.; Du, L. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Li, W.F.; Liu, Y.; Wang, Q.; Cheng, X.L.; Wei, F.; Wang, A.G.; Jin, H.T.; Ma, S.C. Acute toxicity screening of different extractions, components and constituents of Polygonum multiflorum Thunb. on zebrafish (Danio rerio) embryos in vivo. Biomed. Pharm. 2018, 99, 205–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Zhang, M.; Diao, T.; Tang, J.; Dai, M.; Chen, S.; Lin, G. Metabonomics study on Polygonum multiflorum induced liver toxicity in rats by GC-MS. Int. J. Clin. Exp. Med. 2015, 8, 10986–10992. [Google Scholar]
- Lv, G.P.; Meng, L.Z.; Han, D.Q.; Li, H.Y.; Zhao, J.; Li, S.P. Effect of sample preparation on components and liver toxicity of Polygonum multiflorum. J. Pharm. Biomed. Anal. 2015, 109, 105–111. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Huang, Q.; Fang, D.; Li, G.; Zhang, G. Toxicity of raw and processed roots of Polygonum multiflorum. Fitoterapia 2012, 83, 469–475. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Song, C.; Yin, Z.; Chen, Z.; Jia, R.; Liang, X.; Li, L.; Zou, Y.; He, C.; et al. Acute and subchronic toxicity as well as evaluation of safety pharmacology of traditional Chinese medicine “Huhezi”. Int. J. Clin. Exp. Med. 2015, 8, 14553–14564. [Google Scholar]
- Cai, Y.; Gao, Y.; Tan, G.; Wu, S.; Dong, X.; Lou, Z.; Zhu, Z.; Chai, Y. Myocardial lipidomics profiling delineate the toxicity of traditional Chinese medicine Aconiti Lateralis radix praeparata. J. Ethnopharmacol. 2013, 147, 349–356. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, Y.S.; Kim, N.H.; Lee, J.; Jang, D.S.; Kim, J.S. Extract of Cassiae Semen and its major compound inhibit S100b-induced TGF-beta1 and fibronectin expression in mouse glomerular mesangial cells. Eur. J. Pharmacol. 2010, 641, 7–14. [Google Scholar] [CrossRef]
- Fu, F.; Tian, F.; Zhou, H.; Lv, W.; Tie, R.; Ji, L.; Li, R.; Shi, Z.; Yu, L.; Liang, X.; et al. Semen cassiae attenuates myocardial ischemia and reperfusion injury in high-fat diet streptozotocin-induced type 2 diabetic rats. Am. J. Chin. Med. 2014, 42, 95–108. [Google Scholar] [CrossRef]
- Diao, Y.L.; Wang, R.F.; Zhang, C.; Chen, J.X.; Liu, B. A new comprehensive procedure for the quality control of Semen Cassiae and its application in evaluating commercially available material in China. Chin. J. Nat. Med. 2013, 11, 433–441. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Yang, C.; Zhang, X.; Wang, W.; Du, X.; Wang, Q.; Ni, J. Cassiae semen: A review of its phytochemistry and pharmacology (Review). Mol. Med. Rep. 2017, 16, 2331–2346. [Google Scholar] [CrossRef]
- Xu, L.; Chan, C.O.; Lau, C.C.; Yu, Z.; Mok, D.K.; Chen, S. Simultaneous determination of eight anthraquinones in Semen Cassiae by HPLC-DAD. Phytochem. Anal. 2012, 23, 110–116. [Google Scholar] [CrossRef]
- Cao, L.J.; Miao, J.; Liu, J.X.; Gao, W.Y.; Li, X. Research on contents of anthraquinones in Cassiae Semen by principal component analysis. Zhongguo Zhong Yao Za Zhi 2015, 40, 2589–2593. [Google Scholar]
- Pei, Y.; Wei, R.; Sun, J.; Gao, H. Safety assessment of freeze-dried powdered Cassiae Semen: Evaluation of chronic toxicity (26-week) in Sprague-Dawley rats. Toxicol. Rep. 2017, 4, 143–150. [Google Scholar] [CrossRef]
- Kwon, K.S.; Lee, J.H.; So, K.S.; Park, B.K.; Lim, H.; Choi, J.S.; Kim, H.P. Aurantio-obtusin, an anthraquinone from cassiae semen, ameliorates lung inflammatory responses. Phytother. Res. 2018, 32, 1537–1545. [Google Scholar] [CrossRef]
- Kim, M.; Lim, S.J.; Lee, H.J.; Nho, C.W. Cassia tora Seed Extract and Its Active Compound Aurantio-obtusin Inhibit Allergic Responses in IgE-Mediated Mast Cells and Anaphylactic Models. J. Agric. Food Chem. 2015, 63, 9037–9046. [Google Scholar] [CrossRef]
- Yu, X.; Wei, L.H.; Zhang, J.K.; Chen, T.R.; Jin, Q.; Wang, Y.N.; Zhang, S.J.; Dou, T.Y.; Cao, Y.F.; Guo, W.Z.; et al. Anthraquinones from Cassiae semen as thrombin inhibitors: In vitro and in silico studies. Phytochemistry 2019, 165, 112025. [Google Scholar] [CrossRef]
- Guo, R.; Wu, H.; Yu, X.; Xu, M.; Zhang, X.; Tang, L.; Wang, Z. Simultaneous Determination of Seven Anthraquinone Aglycones of Crude and Processed Semen Cassiae Extracts in Rat Plasma by UPLC-MS/MS and Its Application to a Comparative Pharmacokinetic Study. Molecules 2017, 22, 1803. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Lv, X.; Liao, L.; Yang, W.; Li, S.; Lu, P.; Zhu, D. Aurantio-obtusin relaxes systemic arteries through endothelial PI3K/AKT/eNOS-dependent signaling pathway in rats. J. Pharm. Sci. 2015, 128, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.L.; Zhao, D.S.; Fan, Y.X.; Yu, Q.; Lai, Y.S.; Li, P.; Li, H.J. Transcriptome analysis to assess the cholestatic hepatotoxicity induced by Polygoni Multiflori Radix: Up-regulation of key enzymes of cholesterol and bile acid biosynthesis. J. Proteom. 2018, 177, 40–47. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Cao, F.; Liu, P.; Bao, H.; Yan, Y.; Dong, X.; Wang, D.; Wang, Z.; Gong, P. Modulation of transport and metabolism of bile acids and bilirubin by chlorogenic acid against hepatotoxicity and cholestasis in bile duct ligation rats: Involvement of SIRT1-mediated deacetylation of FXR and PGC-1alpha. J. Hepatobiliary Pancreat. Sci. 2018, 25, 195–205. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, D.; Yuan, Z.; Jiang, Z.; Zhou, W.; Li, Z.; Yin, M.; Zhou, Z.; Zhang, L.; Wang, T. 8-Methoxypsoralen disrupts MDR3-mediated phospholipids efflux and bile acid homeostasis and its relevance to hepatotoxicity. Toxicology 2017, 386, 40–48. [Google Scholar] [CrossRef]
- Zhao, D.S.; Jiang, L.L.; Fan, Y.X.; Dong, L.C.; Ma, J.; Dong, X.; Xu, X.J.; Li, P.; Li, H.J. Identification of urine tauro-beta-muricholic acid as a promising biomarker in Polygoni Multiflori Radix-induced hepatotoxicity by targeted metabolomics of bile acids. Food Chem. Toxicol. 2017, 108, 532–542. [Google Scholar] [CrossRef]
- Wu, Z.T.; Qi, X.M.; Sheng, J.J.; Ma, L.L.; Ni, X.; Ren, J.; Huang, C.G.; Pan, G.Y. Timosaponin A3 induces hepatotoxicity in rats through inducing oxidative stress and down-regulating bile acid transporters. Acta Pharm. Sin. 2014, 35, 1188–1198. [Google Scholar] [CrossRef]
- Liu, A.; Krausz, K.W.; Fang, Z.Z.; Brocker, C.; Qu, A.; Gonzalez, F.J. Gemfibrozil disrupts lysophosphatidylcholine and bile acid homeostasis via PPARalpha and its relevance to hepatotoxicity. Arch. Toxicol. 2014, 88, 983–996. [Google Scholar] [CrossRef]
- Sugano, M.; Cho, S.; Imaizumi, K.; Wada, M. Hepatotoxicity and lipid metabolism. 3. Changes in phosphatidylcholine and phosphatidylethanolamine during hepatic injury caused by carbon tetrachloride. Biochem. Pharm. 1970, 19, 2325–2333. [Google Scholar]
- Yousefi-Manesh, H.; Shirooie, S.; Partoazar, A.; Nikoui, V.; Estakhri, M.R.A.; Bakhtiarian, A. Hepatoprotective effects of phosphatidylserine liposomes on carbon tetrachloride-induced hepatotoxicity in rats. J. Cell. Biochem. 2019, 120, 11853–11858. [Google Scholar] [CrossRef]
- Lutkewitte, A.J.; Schweitzer, G.G.; Kennon-McGill, S.; Clemens, M.M.; James, L.P.; Jaeschke, H.; Finck, B.N.; McGill, M.R. Lipin deactivation after acetaminophen overdose causes phosphatidic acid accumulation in liver and plasma in mice and humans and enhances liver regeneration. Food Chem. Toxicol. 2018, 115, 273–283. [Google Scholar] [CrossRef]
- Gaveglio, V.L.; Pasquare, S.J.; Giusto, N.M. Phosphatidic acid metabolism in rat liver cell nuclei. FEBS Lett. 2013, 587, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Gerle, C. On the structural possibility of pore-forming mitochondrial FoF1 ATP synthase. Biochim. Biophys. Acta 2016, 1857, 1191–1196. [Google Scholar] [CrossRef]
- Németi, B.; Regonesi, M.E.; Tortora, P.; Gregus, Z. Polynucleotide phosphorylase and mitochondrial ATP synthase mediate reduction of arsenate to the more toxic arsenite by forming arsenylated analogues of ADP and ATP. Toxicol. Sci. 2010, 117, 270–281. [Google Scholar] [CrossRef]
- Liu, J.; Takase, I.; Hakucho, A.; Okamura, N.; Fujimiya, T. Carvedilol attenuates the progression of alcohol fatty liver disease in rats. Alcohol. Clin. Exp. Res. 2012, 36, 1587–1599. [Google Scholar] [CrossRef]
- Xie, J.; Dong, W.; Liu, R.; Wang, Y.; Li, Y. Research on the hepatotoxicity mechanism of citrate-modified silver nanoparticles based on metabolomics and proteomics. Nanotoxicology 2018, 12, 18–31. [Google Scholar] [CrossRef]
- Teclebrhan, H.; Olsson, J.; Swiezewska, E.; Dallner, G. Biosynthesis of the side chain of ubiquinone:trans-prenyltransferase in rat liver microsomes. J. Biol. Chem. 1993, 268, 23081–23086. [Google Scholar]
- Denk, G.U.; Maitz, S.; Wimmer, R.; Rust, C.; Invernizzi, P.; Ferdinandusse, S.; Kulik, W.; Fuchsbichler, A.; Fickert, P.; Trauner, M.; et al. Conjugation is essential for the anticholestatic effect of NorUrsodeoxycholic acid in taurolithocholic acid-induced cholestasis in rat liver. Hepatology 2010, 52, 1758–1768. [Google Scholar] [CrossRef]
- Beuers, U.; Denk, G.U.; Soroka, C.J.; Wimmer, R.; Rust, C.; Paumgartner, G.; Boyer, J.L. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol 3-kinase-dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J. Biol. Chem. 2003, 278, 17810–17818. [Google Scholar] [CrossRef]
- Kupke, T.; Hernandez-Acosta, P.; Steinbacher, S.; Culianez-Macia, F.A. Arabidopsis thaliana flavoprotein AtHAL3a catalyzes the decarboxylation of 4’-Phosphopantothenoylcysteine to 4’-phosphopantetheine, a key step in coenzyme A biosynthesis. J. Biol. Chem. 2001, 276, 19190–19196. [Google Scholar] [CrossRef]
- Yang, Y.; Piao, X.; Zhang, M.; Wang, X.; Xu, B.; Zhu, J.; Fang, Z.; Hou, Y.; Lu, Y.; Yang, B. Bioactivity-guided fractionation of the triglyceride-lowering component and in vivo and in vitro evaluation of hypolipidemic effects of Calyx seu Fructus Physalis. Lipids Health Dis. 2012, 11, 38. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |










| Group | Body Weight/g | Liver | |
|---|---|---|---|
| Weight/g | W/W*100(%) | ||
| 0 mg/kg | 339 ± 13.41 | 11.56 ± 1.36 | 3.41 ± 0.25 |
| 4 mg/kg | 344 ± 11.54 | 12.18 ± 0.40 | 3.54 ± 0.34 |
| 40 mg/kg | 334 ± 9.77 | 12.19 ± 0.51 | 3.65 ± 0.14 * |
| 200 mg/kg | 335 ± 8.32 | 13.71 ± 0.85 ** | 4.09 ± 0.26 *** |
| Control | Aurantio-obtusin Concentration | |||
|---|---|---|---|---|
| — | 4 mg/kg | 40 mg/kg | 200 mg/kg | |
| WBC (109/L) | 5.44 ± 1.48 | 3.77 ± 0.90 * | 3.08 ± 0.72 ** | 4.66 ± 1.75 |
| RBC (1012/L) | 8.47 ± 0.38 | 8.52 ± 0.38 | 8.10 ± 0.36 | 8.11 ± 0.37 |
| HGB (g/dL) | 167.88 ± 6.85 | 166.38 ± 8.43 | 159.86 ± 5.17 * | 157.67 ± 5.56 ** |
| HCT (%) | 47.93 ± 1.70 | 47.24 ± 2.00 | 45.67 ± 1.51 * | 45.32 ± 1.58 ** |
| PLT (109/L) | 950.38 ± 97.89 | 953.50 ± 150.34 | 940.14 ± 90.91 | 981.00 ± 170.57 |
| MPV (fL) | 7.15 ± 0.24 | 7.13 ± 0.22 | 7.14 ± 0.18 | 7.28 ± 0.09 |
| PCT (%) | 0.68 ± 0.08 | 0.68 ± 0.11 | 0.67 ± 0.06 | 0.72 ± 0.12 |
| MCV (fL) | 56.63 ± 1.33 | 55.48 ± 1.47 | 56.40 ± 0.98 | 55.93 ± 1.77 |
| MCH (pg) | 19.84 ± 0.38 | 19.56 ± 0.54 | 19.76 ± 0.36 | 19.45 ± 0.53 |
| MCHC (g/dL) | 350.13 ± 4.65 | 352.38 ± 4.15 | 350.00 ± 2.39 | 347.67 ± 4.50 |
| NEUT (%) | 7.48 ± 2.83 | 15.49 ± 8.68 * | 7.33 ± 2.44 | 6.09 ± 2.29 |
| LYMPH (%) | 90.14 ± 2.73 | 81.38 ± 8.75 * | 90.13 ± 3.02 | 91.67 ± 2.63 |
| MONO (%) | 1.75 ± 0.83 | 2.25 ± 0.54 | 1.87 ± 0.60 | 1.60 ± 0.89 |
| EO (%) | 0.63 ± 0.27 | 0.86 ± 0.37 | 0.67 ± 0.38 | 0.65 ± 0.31 |
| BASO (%) | 0.01 ± 0.03 | 0.03 ± 0.07 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| NEUT# | 0.39 ± 0.14 | 0.59 ± 0.30 | 0.23 ± 0.10 * | 1.21 ± 1.44 |
| LYMPH# | 4.92 ± 1.40 | 3.06 ± 0.78 ** | 2.77 ± 0.62 ** | 3.33 ± 0.48 ** |
| MONO# | 0.09 ± 0.04 | 0.09 ± 0.03 | 0.06 ± 0.03 | 0.09 ± 0.08 |
| EO# | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.02 ± 0.02 | 0.03 ± 0.02 |
| BASO# | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| PDW (%) | 8.00 ± 0.30 | 7.91 ± 0.29 | 7.73 ± 0.17 * | 8.02 ± 0.27 |
| RET# | 0.10 ± 0.02 | 0.09 ± 0.03 | 0.09 ± 0.02 | 0.10 ± 0.02 |
| RET (%) | 1.22 ± 0.26 | 1.07 ± 0.34 | 1.07 ± 0.22 | 1.23 ± 0.24 |
| Control | Aurantio-Obtusin | |||
|---|---|---|---|---|
| — | 4 mg/kg | 40 mg/kg | 200 mg/kg | |
| ALT (U/L) | 30.17 ± 5.34 | 32.50 ± 3.86 | 62.33 ± 5.76 *** | 85.67 ± 7.13 *** |
| AST (U/L) | 89.20 ± 10.93 | 115.17 ± 10.40 *** | 189.63 ± 22.11 *** | 223.17 ± 16.34 *** |
| ALP (U/L) | 233.17 ± 23.05 | 288.67 ± 41.52 ** | 353.33 ± 44.43 *** | 560.83 ± 60.88 *** |
| TP (g/L) | 44.22 ± 3.85 | 35.64 ± 2.91 *** | 36.70 ± 4.23 *** | 33.25 ± 4.45 *** |
| ALB (g/L) | 22.40 ± 1.90 | 19.75 ± 1.16 ** | 19.35 ± 1.84 ** | 17.43 ± 2.20 *** |
| TCHO (mmol/L) | 1.48 ± 0.15 | 1.17 ± 0.07 *** | 1.11 ± 0.08 *** | 1.00 ± 0.13 *** |
| Ion Mode | TR_m/z | RSD (%) | |
|---|---|---|---|
| TR | Area | ||
| Positive | 1.98_317.0694 | 0.22 | 2.08 |
| 5.75_620.2559 | 0 | 3.82 | |
| 6.10_240.1049 | 0.21 | 5.23 | |
| 8.01_323.2295 | 0 | 2.54 | |
| 11.68_369.3548 | 0.04 | 5.25 | |
| Negative | 3.33_221.1546 | 0.08 | 3.05 |
| 5.70_630.3229 | 0.14 | 2.68 | |
| 6.48_481.3134 | 0.12 | 3.34 | |
| 7.43_628.2766 | 0.16 | 5.52 | |
| 11.37_624.4236 | 0 | 8.96 | |
| Mode | RT/min | m/z | Formula | Biomarker | HMDB | KEGG | Trend | ppm |
|---|---|---|---|---|---|---|---|---|
| ESI+ | 5.75 | 240.1046 | C7H17N3O4S | N-Ornithyl-l-taurine | 033519 | Unknown | ↓ | 5 |
| 5.44 | 520.3421 | C26H50NO7P | LysoPC (18:2) | 010386 | Unknown | ↑ | 0 | |
| 0.62 | 74.0684 | C3H7NO | N,N-Dimethylformamide | 001888 | C03134 | ↓ | 1 | |
| 12.87 | 778.5466 | C44H76NO8P | PC (22:5/14:1) | 008657 | C00157 | ↓ | 2 | |
| 12.94 | 319.198 | C19H26O4 | Ubiquinone-2 | 006709 | C00399 | ↓ | 1 | |
| 6.1 | 185.0824 | C9H12O4 | Vanylglycol | 001490 | C05594 | ↑ | 5 | |
| 6.94 | 794.5065 | C43H72NO10P | PS (15:0/22:6) | 112339 | Unknown | ↑ | 4 | |
| 8.41 | 524.3724 | C26H54NO7P | LysoPC (18:0) | 010384 | C04230 | ↑ | 2 | |
| 13.12 | 811.6233 | C47H87O8P | PA (24:1/20:2) | 115604 | Unknown | ↑ | 1 | |
| 12.79 | 812.6262 | C46H86NO8P | PC (20:0/18:3) | 008271 | C00157 | ↑ | 4 | |
| 0.59 | 160.0674 | C6H9NO4 | l-2-Amino-4-methylenepentanedioic acid | 029433 | C00651 | ↓ | 3 | |
| ESI- | 12.86 | 883.5334 | C47H81O13P | PI (22:5/16:0) | 009918 | C00626 | ↑ | 5 |
| 12.87 | 803.5625 | C47H81O8P | PA (22:1/22:5) | 115292 | Unknown | ↓ | 0 | |
| 0.56 | 313.066 | C10H20O7P2 | Geranyl-PP | 001285 | C05847 | ↓ | 4 | |
| 5.75 | 507.3172 | C25H49O8P | PA (8:0/i-14:0) | 115687 | Unknown | ↓ | 2 | |
| 8.01 | 508.3416 | C25H52NO7P | LysoPC (17:0) | 012108 | C04230 | ↓ | 2 | |
| 12.85 | 723.5049 | C41H73O8P | PA (16:0/22:4) | 114846 | Unknown | ↑ | 4 | |
| 8.34 | 480.3101 | C23H48NO7P | LysoPC (15:0) | 010381 | C04230 | ↓ | 1 | |
| 5.72 | 504.3108 | C25H48NO7P | LysoPE (20:2/0:0) | 011513 | Unknown | ↓ | 1 | |
| 6.42 | 658.3331 | C32H53NO11S | Taurolithocholic acid 3-glucuronide | 002429 | Unknown | ↓ | 5 | |
| 5.7 | 552.3101 | C29H48NO7P | LysoPE (0:0/24:6) | 011499 | Unknown | ↓ | 1 | |
| 2.61 | 401.0877 | C12H23N2O9PS | 4′-Phosphopantothenoylcysteine | 001117 | C04352 | ↓ | 3 | |
| 12.88 | 857.6731 | C56H90O6 | TG (15:0/18:4/20:5) | 043681 | Unknown | ↓ | 4 | |
| 1.94 | 407.2812 | C24H40O5 | Cholic acid | 000619 | C00695 | ↑ | 2 | |
| 6.97 | 464.3017 | C26H43NO6 | Glycocholic acid | 000138 | C01921 | ↑ | 0 | |
| 5.08 | 498.2874 | C26H45NO6S | Taurodeoxycholic acid | 000896 | C05463 | ↓ | 4 | |
| 3.19 | 391.2853 | C24H40O4 | Deoxycholic acid | 000626 | C04483 | ↑ | 0 | |
| 2.32 | 514.2822 | C26H45NO7S | Taurocholic acid | 000036 | C05122 | ↓ | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Li, J.; Tang, X.; Wang, Y.; Ma, Z.; Gao, Y. Metabolomics of Aurantio-Obtusin-Induced Hepatotoxicity in Rats for Discovery of Potential Biomarkers. Molecules 2019, 24, 3452. https://doi.org/10.3390/molecules24193452
Xu L, Li J, Tang X, Wang Y, Ma Z, Gao Y. Metabolomics of Aurantio-Obtusin-Induced Hepatotoxicity in Rats for Discovery of Potential Biomarkers. Molecules. 2019; 24(19):3452. https://doi.org/10.3390/molecules24193452
Chicago/Turabian StyleXu, Longlong, Jian Li, Xianglin Tang, Yuguang Wang, Zengchun Ma, and Yue Gao. 2019. "Metabolomics of Aurantio-Obtusin-Induced Hepatotoxicity in Rats for Discovery of Potential Biomarkers" Molecules 24, no. 19: 3452. https://doi.org/10.3390/molecules24193452
APA StyleXu, L., Li, J., Tang, X., Wang, Y., Ma, Z., & Gao, Y. (2019). Metabolomics of Aurantio-Obtusin-Induced Hepatotoxicity in Rats for Discovery of Potential Biomarkers. Molecules, 24(19), 3452. https://doi.org/10.3390/molecules24193452