Tuning Gel State Properties of Supramolecular Gels by Functional Group Modification
Abstract
:1. Introduction
2. Results
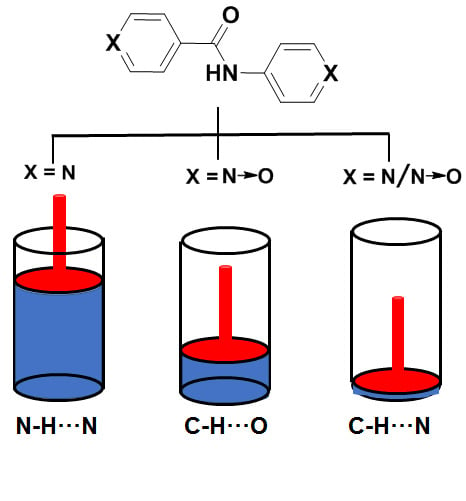
2.1. Design and Synthesis
2.2. Gelation Experiments
2.3. Thermal Stability
2.4. Rheology
2.5. Scanning Electron Microscopy (SEM)
2.6. Crystal Structure
2.7. X-ray Powder Diffraction (XRPD)
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Synthesis
4.3. Gelation Studies
4.4. Minimum Gel Concentration (MGC)
4.5. Tgel Experiments
4.6. Rheology
4.7. Scanning Electron Microscopy (SEM)
4.8. Crystallography
4.9. X-ray Powder Diffraction (XRPD)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- De Loos, M.; Feringa, B.L.; van Esch, J.H. Design and Application of Self-Assembled Low Molecular Weight Hydrogels. Eur. J. Org. Chem. 2005, 2005, 3615–3631. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular Organogels. Soft Matter Comprised of Low-Molecular-Mass Organic Gelators and Organic Liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Steed, J.W. Anion-tuned supramolecular gels: A natural evolution from urea supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 3686–3699. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef]
- Kumar, D.K.; Steed, J.W. Supramolecular gel phase crystallization: Orthogonal self-assembly under non-equilibrium conditions. Chem. Soc. Rev. 2014, 43, 2080–2088. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, R.K.; Maitra, U. Supramolecular gels ‘in action’. J. Mater. Chem. 2009, 19, 6649–6687. [Google Scholar] [CrossRef]
- Foster, J.A.; Damodaran, K.K.; Maurin, A.; Day, G.M.; Thompson, H.P.G.; Cameron, G.J.; Bernal, J.C.; Steed, J.W. Pharmaceutical polymorph control in a drug-mimetic supramolecular gel. Chem. Sci. 2017, 8, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Worthington, P.; Pochan, D.J.; Langhans, S.A. Peptide Hydrogels—Versatile Matrices for 3D Cell Culture in Cancer Medicine. Front. Oncol. 2015, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Truong, W.T.; Su, Y.; Meijer, J.T.; Thordarson, P.; Braet, F. Self-Assembled Gels for Biomedical Applications. Chem. Asian J. 2011, 6, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Wang, R.-Y.; Liu, X.-Y.; Pan, H.-H. Nanoengineering of a Biocompatible Organogel by Thermal Processing. J. Phys. Chem. B 2009, 113, 5011–5015. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Liu, X.-Y.; Wang, R.-Y.; Xiong, J.-Y. Architecture of a Biocompatible Supramolecular Material by Supersaturation-Driven Fabrication of its Fiber Network. J. Phys. Chem. B 2005, 109, 24231–24235. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, R.; Kraatz, H.-B. Sonication-Induced Coiled Fibrous Architectures of Boc-L-Phe-L-Lys(Z)-OMe. Chem. Eur. J. 2013, 19, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Dhibar, S.; Dey, A.; Majumdar, S.; Ghosh, D.; Mandal, A.; Ray, P.P.; Dey, B. A supramolecular Cd(ii)-metallogel: An efficient semiconductive electronic device. Dalton Trans. 2018, 47, 17412–17420. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-Y.; Liu, X.-Y.; Li, J.-L. Engineering Molecular Self-Assembled Fibrillar Networks by Ultrasound. Cryst. Growth Des. 2009, 9, 3286–3291. [Google Scholar] [CrossRef]
- Alexander, S.L.M.; Korley, L.T.J. Nucleation effects of high molecular weight polymer additives on low molecular weight gels. Polym. J. 2018, 50, 775–786. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Liu, X.-Y.; Narayanan, J.; Xiong, J.-Y.; Li, J.-L. Architecture of Fiber Network: From Understanding to Engineering of Molecular Gels. J. Phys. Chem. B 2006, 110, 25797–25802. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. How should multicomponent supramolecular gels be characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Meng, L.; Li, Z.; Mao, Y.; Lan, H.; Chen, L.; Fan, Y.; Yi, T. Large Red-Shifted Fluorescent Emission via Intermolecular π–π Stacking in 4-Ethynyl-1,8-naphthalimide-Based Supramolecular Assemblies. Langmuir 2014, 30, 11753–11760. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, D.; Zhu, D. A Chiral Low-Molecular-Weight Gelator Based on Binaphthalene with Two Urea Moieties: Modulation of the CD Spectrum after Gel Formation. Langmuir 2007, 23, 1478–1482. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Xiang, J.; Zhu, D. New Organogels Based on an Anthracene Derivative with One Urea Group and Its Photodimer: Fluorescence Enhancement after Gelation. Langmuir 2007, 23, 9195–9200. [Google Scholar] [CrossRef]
- Wang, G.; Cheuk, S.; Yang, H.; Goyal, N.; Reddy, P.V.N.; Hopkinson, B. Synthesis and Characterization of Monosaccharide-Derived Carbamates as Low-Molecular-Weight Gelators. Langmuir 2009, 25, 8696–8705. [Google Scholar] [CrossRef]
- Gao, W.; Liang, Y.; Peng, X.; Hu, Y.; Zhang, L.; Wu, H.; He, B. In situ injection of phenylboronic acid based low molecular weight gels for efficient chemotherapy. Biomaterials 2016, 105, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Das, U.K.; Banerjee, S.; Dastidar, P. Remarkable Shape-Sustaining, Load-Bearing, and Self-Healing Properties Displayed by a Supramolecular Gel Derived from a Bis-pyridyl-bis-amide of L-Phenyl Alanine. Chem. Asian J. 2014, 9, 2475–2482. [Google Scholar] [CrossRef]
- Tang, Y.-T.; Dou, X.-Q.; Ji, Z.-A.; Li, P.; Zhu, S.-M.; Gu, J.-J.; Feng, C.-L.; Zhang, D. C2-symmetric cyclohexane-based hydrogels: A rational designed LMWG and its application in dye scavenging. J. Mol. Liq. 2013, 177, 167–171. [Google Scholar] [CrossRef]
- Li, P.; Dou, X.-Q.; Tang, Y.-T.; Zhu, S.; Gu, J.; Feng, C.-L.; Zhang, D. Gelator-polysaccharide hybrid hydrogel for selective and controllable dye release. J. Colloid Interface Sci. 2012, 387, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Lebedytė, I.; Yufit, D.S.; Damodaran, K.K.; Steed, J.W. Selective gelation of N-(4-pyridyl)nicotinamide by copper(ii) salts. CrystEngComm 2015, 17, 8130–8138. [Google Scholar] [CrossRef]
- Kumar, D.K.; Jose, D.A.; Dastidar, P.; Das, A. Nonpolymeric Hydrogelator Derived from N-(4-Pyridyl)isonicotinamide. Langmuir 2004, 20, 10413–10418. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Kumar, D.K.; Raghavan, S.R.; Dastidar, P. Supramolecular Synthons in Designing Low Molecular Mass Gelling Agents: L-Amino Acid Methyl Ester Cinnamate Salts and their Anti-Solvent-Induced Instant Gelation. Chem. Asian J. 2011, 6, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Bhattacharya, S. Role of synergistic [small pi]-[small pi] stacking and X-H[three dots, centered]Cl (X = C, N, O) H-bonding interactions in gelation and gel phase crystallization. Chem. Commun. 2015, 51, 7019–7022. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef]
- Ghosh, D.; Ferfolja, K.; Drabavičius, Ž.; Steed, J.W.; Damodaran, K.K. Crystal habit modification of Cu(ii) isonicotinate–N-oxide complexes using gel phase crystallisation. New J. Chem. 2018, 42, 19963–19970. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Weiss, R.G.; Terech, P. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Heidelberg, Germany, 2006; p. 978. [Google Scholar]
- Fages, F.; Voegtle, F.; Zinic, M. Systematic design of amide- and urea-type gelators with tailored properties. Top. Curr. Chem. 2005, 256, 77–131. [Google Scholar]
- Isare, B.; Pensec, S.; Raynal, M.; Bouteiller, L. Bisurea-based supramolecular polymers: From structure to properties1. C. R. Chim. 2016, 19, 148–156. [Google Scholar] [CrossRef]
- Gale, P.A.; Busschaert, N.; Haynes, C.J.E.; Karagiannidis, L.E.; Kirby, I.L. Anion receptor chemistry: Highlights from 2011 and 2012. Chem. Soc. Rev. 2014, 43, 205–241. [Google Scholar] [CrossRef] [PubMed]
- Dzolic, Z.; Cametti, M.; Dalla Cort, A.; Mandolini, L.; Zinic, M. Fluoride-responsive organogelator based on oxalamide-derived anthraquinone. Chem. Commun. 2007, 34, 3535–3537. [Google Scholar] [CrossRef] [PubMed]
- Kotova, O.; Daly, R.; dos Santos, C.M.G.; Boese, M.; Kruger, P.E.; Boland, J.J.; Gunnlaugsson, T. Europium-Directed Self-Assembly of a Luminescent Supramolecular Gel from a Tripodal Terpyridine-Based Ligand. Angew. Chem. Int. Ed. 2012, 51, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cavicchi, K.A. Investigation of the relationships between the thermodynamic phase behavior and gelation behavior of a series of tripodal trisamide compounds. Soft Matter 2012, 8, 6483–6492. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Ira; Krishnamoorthy, G.; Maitra, U. Dynamics of Bound Dyes in a Nonpolymeric Aqueous Gel Derived from a Tripodal Bile Salt. J. Phys. Chem. B 2003, 107, 2189–2192. [Google Scholar] [CrossRef]
- Li, Z.; Cao, J.; Li, H.; Liu, H.; Han, F.; Liu, Z.; Tong, C.; Li, S. Self-assembled drug delivery system based on low-molecular-weight bis-amide organogelator: Synthesis, properties and in vivo evaluation. Drug Deliv. 2016, 23, 3168–3178. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S.J.; Dee, G.; Kotova, O.; McCoy, C.P.; Gunnlaugsson, T. Luminescent lanthanide (Eu(iii)) cross-linked supramolecular metallo co-polymeric hydrogels: The effect of ligand symmetry. Chem. Commun. 2019, 55, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.K.; Jose, D.A.; Dastidar, P.; Das, A. Nonpolymeric Hydrogelators Derived from Trimesic Amides. Chem. Mater. 2004, 16, 2332–2335. [Google Scholar] [CrossRef]
- Meazza, L.; Foster, J.A.; Fucke, K.; Metrangolo, P.; Resnati, G.; Steed, J.W. Halogen-bonding-triggered supramolecular gel formation. Nat. Chem. 2013, 5, 42–47. [Google Scholar] [CrossRef]
- Pal, A.; Ghosh, Y.K.; Bhattacharya, S. Molecular mechanism of physical gelation of hydrocarbons by fatty acid amides of natural amino acids. Tetrahedron 2007, 63, 7334–7348. [Google Scholar] [CrossRef]
- Kawamoto, K.; Grindy, S.C.; Liu, J.; Holten-Andersen, N.; Johnson, J.A. Dual Role for 1,2,4,5-Tetrazines in Polymer Networks: Combining Diels–Alder Reactions and Metal Coordination to Generate Functional Supramolecular Gels. ACS Macro Lett. 2015, 4, 458–461. [Google Scholar] [CrossRef]
- Das, U.K.; Banerjee, S.; Dastidar, P. Primary Ammonium Monocarboxylate Synthon in Designing Supramolecular Gels: A New Series of Chiral Low-Molecular-Weight Gelators Derived from Simple Organic Salts that are Capable of Generating and Stabilizing Gold Nanoparticles. Chem. Asian J. 2013, 8, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhang, L.; Liu, M. Solvent-Polarity-Tuned Morphology and Inversion of Supramolecular Chirality in a Self-Assembled Pyridylpyrazole-Linked Glutamide Derivative: Nanofibers, Nanotwists, Nanotubes, and Microtubes. Chem. Eur. J. 2013, 19, 9234–9241. [Google Scholar] [CrossRef] [PubMed]
- Karak, S.; Kumar, S.; Bera, S.; Diaz, D.D.; Banerjee, S.; Vanka, K.; Banerjee, R. Interplaying anions in a supramolecular metallohydrogel to form metal organic frameworks. Chem. Commun. 2017, 53, 3705–3708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartha, K.K.; Praveen, V.K.; Babu, S.S.; Cherumukkil, S.; Ajayaghosh, A. Pyridyl-Amides as a Multimode Self-Assembly Driver for the Design of a Stimuli-Responsive π-Gelator. Chem. Asian J. 2015, 10, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Li, W.; Wu, L. Structural Characterization and Chemical Response of a Ag-Coordinated Supramolecular Gel. Langmuir 2007, 23, 8217–8223. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Jung, S.H.; Park, S.; Park, K.-M.; Jung, J.H. A metal-organic framework gel with Cd2+ derived from only coordination bonds without intermolecular interactions and its catalytic ability. New J. Chem. 2013, 37, 2330–2335. [Google Scholar] [CrossRef]
- Aoyama, R.; Sako, H.; Amakatsu, M.; Yamanaka, M. Palladium ion-induced supramolecular gel formation of tris-urea molecules. Polym. J. 2015, 47, 136–140. [Google Scholar] [CrossRef]
- Ghosh, K.; Panja, S.; Bhattacharya, S. Naphthalene linked pyridyl urea as a supramolecular gelator: A new insight into naked eye detection of I- in the gel state with semiconducting behaviour. RSC Adv. 2015, 5, 72772–72779. [Google Scholar] [CrossRef]
- Kumar, D.P. Synthesis of gold nanoparticles and nanoclusters in a supramolecular gel and their applications in catalytic reduction of p-nitrophenol to p-aminophenol and Hg(ii) sensing. RSC Adv. 2014, 4, 45449–45457. [Google Scholar] [CrossRef]
- Byrne, P.; Lloyd, G.O.; Applegarth, L.; Anderson, K.M.; Clarke, N.; Steed, J.W. Metal-induced gelation in dipyridyl ureas. New J. Chem. 2010, 34, 2261–2274. [Google Scholar] [CrossRef]
- Pandurangan, K.; Kitchen, J.A.; Blasco, S.; Paradisi, F.; Gunnlaugsson, T. Supramolecular pyridyl urea gels as soft matter with antibacterial properties against MRSA and/or E. coli. Chem. Commun. 2014, 50, 10819–10822. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.E.; Clarke, N.; Anderson, K.M.; Elder, J.A.; Lenthall, J.T.; Steed, J.W. Anion binding inhibition of the formation of a helical organogel. Chem. Commun. 2006, 30, 3199–3201. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.W.; Hughes, R.W. Rheology for Chemists: An Introduction, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2008; p. 264. [Google Scholar]
- Guenet, J.-M. Rheological Aspects. In Organogels: Thermodynamics, Structure, Solvent Role, and Properties; Guenet, J.-M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 83–94. [Google Scholar]
- Sathaye, S.; Mbi, A.; Sonmez, C.; Chen, Y.; Blair, D.L.; Schneider, J.P.; Pochan, D.J. Rheology of peptide- and protein-based physical hydrogels: Are everyday measurements just scratching the surface? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 34–68. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. B 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Avery, R.K.; Assmann, A.; Paul, A.; McKinley, G.H.; Khademhosseini, A.; Olsen, B.D. Shear-Thinning Nanocomposite Hydrogels for the Treatment of Hemorrhage. ACS Nano 2014, 8, 9833–9842. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, D.; Thierry, A.; Rochas, C.; Ajayaghosh, A.; Guenet, J.M. Key role of solvent type in organogelation. Soft Matter 2012, 8, 8714–8721. [Google Scholar] [CrossRef]
- Colquhoun, C.; Draper, E.R.; Eden, E.G.B.; Cattoz, B.N.; Morris, K.L.; Chen, L.; McDonald, T.O.; Terry, A.E.; Griffiths, P.C.; Serpell, L.C.; et al. The effect of self-sorting and co-assembly on the mechanical properties of low molecular weight hydrogels. Nanoscale 2014, 6, 13719–13725. [Google Scholar] [CrossRef] [Green Version]
- Raeburn, J.; Adams, D.J. Multicomponent low molecular weight gelators. Chem. Commun. 2015, 51, 5170–5180. [Google Scholar] [CrossRef]
- Tómasson, D.A.; Ghosh, D.; Kržišnik, Z.; Fasolin, L.H.; Vicente, A.A.; Martin, A.D.; Thordarson, P.; Damodaran, K.K. Enhanced Mechanical and Thermal Strength in Mixed-Enantiomers-Based Supramolecular Gel. Langmuir 2018, 34, 12957–12967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvée, C.; Ström, A.; Haukka, M.; Sundén, H. A Multi-Component Reaction towards the Development of Highly Modular Hydrogelators. Chem. Eur. J. 2018, 24, 8071–8075. [Google Scholar] [CrossRef]
- Gardner, J.N.; Katritzky, A.R. 875. N-oxides and related compounds. Part V. The tautomerism of 2- and 4-amino- and -hydroxy-pyridine 1-oxide. J. Chem. Soc. 1957, 4375–4385. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71 Pt 1, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds diNO, INO, and PNO are available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, D.; Mulvee, M.T.; Damodaran, K.K. Tuning Gel State Properties of Supramolecular Gels by Functional Group Modification. Molecules 2019, 24, 3472. https://doi.org/10.3390/molecules24193472
Ghosh D, Mulvee MT, Damodaran KK. Tuning Gel State Properties of Supramolecular Gels by Functional Group Modification. Molecules. 2019; 24(19):3472. https://doi.org/10.3390/molecules24193472
Chicago/Turabian StyleGhosh, Dipankar, Matthew T. Mulvee, and Krishna K. Damodaran. 2019. "Tuning Gel State Properties of Supramolecular Gels by Functional Group Modification" Molecules 24, no. 19: 3472. https://doi.org/10.3390/molecules24193472
APA StyleGhosh, D., Mulvee, M. T., & Damodaran, K. K. (2019). Tuning Gel State Properties of Supramolecular Gels by Functional Group Modification. Molecules, 24(19), 3472. https://doi.org/10.3390/molecules24193472