Mapping the Biotransformation of Coumarins through Filamentous Fungi
Abstract
:1. Introduction
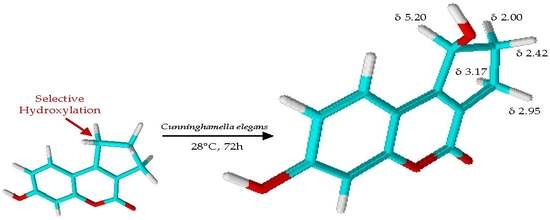
2. Results and Discussion
3. Materials and Methods
3.1. General Analytical Procedures
3.2. Substrates
3.3. Biotransformation Assays
3.4. Isolation and Purification of the Derivatives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; Intech: London, UK, 2015; pp. 113–140. [Google Scholar]
- Bone, K.; Mills, S. Principles and Practice of Phytotherapy—Modern Herbal Medicine, 2nd ed.; Elsevier: Atlanta, GA, USA, 2013; p. 1076. [Google Scholar]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.M.; Monache, F.D.; Smânia, A.J. Antibacterial activity of coumarins. Z. Naturforsch. 2005, 60, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- Capra, J.C.; Cunha, M.P.; Machado, D.G.; Zomkowski, A.D.E.; Mendes, B.G.; Santos, A.R.S.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like effect of scopoletin, a coumarin isolated from Polygala sabulosa (Polygalaceae) in mice: Evidence for the involvement of monoaminergic systems. Eur. J. Pharmacol. 2010, 643, 232–238. [Google Scholar] [CrossRef]
- Kawase, M.; Sakagami, H.; Motohashi, N.; Hauer, H.; Chatterjee, S.S.; Spengler, G.; Vigyikanne, A.V.; Molnár, A.; Molnár, J. Coumarin derivatives with tumor-specific cytotoxicity and multidrug resistance reversal activity. In Vivo 2005, 19, 705–712. [Google Scholar]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Mira, A.; Alkhiary, W.; Zhu, Q.; Nakagawa, T.; Tran, H.B.; Amen, Y.M.; Shimizu, K. Improved biological activities of isoepoxypteryxin by biotransformation. Chem. Biodivers. 2016, 13, 1307–1315. [Google Scholar] [CrossRef]
- Yang, X.; Hou, J.; Liu, D.; Deng, S.; Wang, Z.B.; Kuang, H.X.; Wang, C.; Yao, J.H.; Liu, K.X.; Ma, X.C. Biotransformation of isoimperatorin by Cunninghamella blakesleana AS 3.970. J. Mol. Catal. B Enzym. B 2013, 88, 1–6. [Google Scholar] [CrossRef]
- Vargas-Soto, F.A.; Céspedes-Acuña, C.L.; Aqueveque- Muñoz, P.M.; Alarcón-Enos, J.E. Toxicity of coumarins synthesized by Pechmann-Duisberg condensation against Drosophila melanogaster larvae and antibacterial effects. Food Chem. Toxicol. 2017, 109, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Zareyee, D.; Serehneh, M. Recyclable CMK-5 supported sulfonic acid as an environmentally benign catalyst for solvent-free one-pot construction of coumarin through Pechmann condensation. J. Mol. Catal. A Chem. 2014, 391, 88–91. [Google Scholar] [CrossRef]
- Maheswara, M.; Siddaiah, V.; Damu, G.L.V.; Rao, Y.K.; Rao, C.V. A solvent-free synthesis of coumarins via Pechmann condensation using heterogeneous catalyst. J. Mol. Catal. A Chem. 2006, 255, 49–52. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Recent advances in the synthesis of coumarin derivatives via Knoevenagel condensation: A review. Synth. Commun. Rev. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Watson, W.J.W. How do the fine chemical, pharmaceutical, and related industries approach green chemistry and sustainability? Green Chem. 2012, 14, 251–259. [Google Scholar] [CrossRef]
- Hai-Feng, Z.; Guo-Qing, H.; Jing, L.; Hui, R.; Qi-He, C.; Qiang, Z.; Jin-Ling, W.; Hong-Bo, Z. Production of gastrodin through biotransformation of p-2-hydroxybenzyl alcohol by cultured cells of Armillaria luteo-virens Sacc. Enzyme Microb. Technol. 2008, 43, 25–30. [Google Scholar] [CrossRef]
- Müller, M. Chemical diversity through biotransformations. Curr. Opin. Biotechnol. 2004, 15, 591–598. [Google Scholar] [CrossRef]
- Silva, E.O.; Furtado, N.A.J.C.; Aleu, J.; Collado, I.G. Non-terpenoid biotransformations by Mucor species. Phytochem. Rev. 2015, 14, 745–764. [Google Scholar] [CrossRef]
- Fraga, B.M.; Gonzalez-vallejo, V.; Guillermo, R. On the biotransformation of ent-trachylobane to ent-kaur-11-ene diterpenes. J. Nat. Prod. 2011, 74, 1985–1989. [Google Scholar] [CrossRef]
- Straathof, A.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2002, 13, 548–556. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Rhoden, S.A.; Mota, T.R.; Azevedo, J.L.; Pamphile, J.A.; de Souza, C.G.M.; de Moraes, M.D.L.T.; Bracht, A.; Peralta, R.M. Endophytic fungi: Expanding the arsenal of industrial enzyme producers. J. Ind. Microbiol. Biotechnol. 2014, 41, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, J.S.; Conceição, J.C.S.; de Oliveira Silva, E. Biotransformation of coumarins by filamentous fungi: An alternative way for achievement of bioactive analogs. Mini. Rev. Org. Chem. 2019, 16, 568–577. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, E.T.; Chun, S.C.; Keum, Y.S. Biotransformation of isoflavones by Aspergillus niger and Cunninghamella elegans. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 523–527. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, 5th ed.; Cengage Learning: Boston, MA, USA, 2014; p. 784. [Google Scholar]
- Fraga, B.M.; Guillermo, R.; Herna, M.G.; Garbarino, J.A. Biotransformation of two stemodane diterpenes by Mucor plumbeus. Tetrahedron 2004, 60, 7921–7932. [Google Scholar] [CrossRef]
- Dubovik, I.P.; Garazd, M.M.; Khilya, V.P. Modified coumarins. 14. Synthesis of 7-hydroxy-[4,3′]dichromenyl-2, 2′-dione derivatives. Chem. Nat. Compd. 2004, 40, 434–443. [Google Scholar] [CrossRef]
- Molnar, M.; Šarkanj, B.; Čačić, M.; Gille, L.; Strelec, I. Antioxidant properties and growth-inhibitory activity of coumarin Schiff bases against common foodborne fungi. Der Pharma Chem. 2014, 6, 313–320. [Google Scholar]
- Molnar, M.; Tomić, M.; Pavić, V. Coumarinyl thiosemicarbazides as antimicrobial agents. Pharm. Chem. J. 2018, 51, 1078–1081. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, G.; Han, X.; Bao, G.; Wang, L.; Zhai, X.; Gong, P. Synthesis and biological evaluation of benzothiazole derivatives bearing the ortho-hydroxy-N-acylhydrazone moiety as potent antitumor agents. Arch. Pharm. (Weinh.) 2014, 347, 936–949. [Google Scholar] [CrossRef]
- Prateeptongkum, S.; Duangdee, N.; Thongyoo, P. Facile iron(III) chloride hexahydrate catalyzed synthesis of coumarins. ARKIVOC 2015, 2015, 248–258. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Shao, J.; Peng, Q.; Zhang, W.; Ma, L.; Chan, A.S.C.; Gu, L. Hydroxycoumarin derivatives: Novel and potent α-glucosidase inhibitors. J. Med. Chem. 2010, 53, 8252–8259. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, M.; Zhou, L.; Zhu, J.; Guan, S.; Yu, R. Biotransformation of 3,4-cyclocondensed coumarins by transgenic hairy roots of Polygonum multiflorum. Afr. J. Pharm. Pharmacol. 2012, 6, 3047–3054. [Google Scholar] [CrossRef]
- Lizzul-Jurse, A.; Bailly, L.; Hubert-Roux, M.; Afonso, C.; Renard, P.Y.; Sabot, C. Readily functionalizable phosphonium-tagged fluorescent coumarins for enhanced detection of conjugates by mass spectrometry. Org. Biomol. Chem. 2016, 14, 7777–7791. [Google Scholar] [CrossRef] [PubMed]
- Smitha, G.; Reddy, C.S. ZrCl4-catalyzed Pechmann reaction: Synthesis of coumarins under solvent-free conditions. Synth. Commun. 2004, 34, 3997–4003. [Google Scholar] [CrossRef]
- Dubovik, I.P.; Garazd, M.M.; Khilya, V.P. Modified coumarins. 14. Synthesis of 7-hydroxy-[4,3′]dichromenyl-2,2′-dione derivatives. Chem. Nat. Compd. 2004, 40, 358–365. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, J.S.d.; Núñez, W.E.R.; Santos, V.H.P.d.; Aleu, J.; Cunha, S.; Silva, E.d.O. Mapping the Biotransformation of Coumarins through Filamentous Fungi. Molecules 2019, 24, 3531. https://doi.org/10.3390/molecules24193531
Nascimento JSd, Núñez WER, Santos VHPd, Aleu J, Cunha S, Silva EdO. Mapping the Biotransformation of Coumarins through Filamentous Fungi. Molecules. 2019; 24(19):3531. https://doi.org/10.3390/molecules24193531
Chicago/Turabian StyleNascimento, Jainara Santos do, Wilson Elias Rozo Núñez, Valmore Henrique Pereira dos Santos, Josefina Aleu, Sílvio Cunha, and Eliane de Oliveira Silva. 2019. "Mapping the Biotransformation of Coumarins through Filamentous Fungi" Molecules 24, no. 19: 3531. https://doi.org/10.3390/molecules24193531
APA StyleNascimento, J. S. d., Núñez, W. E. R., Santos, V. H. P. d., Aleu, J., Cunha, S., & Silva, E. d. O. (2019). Mapping the Biotransformation of Coumarins through Filamentous Fungi. Molecules, 24(19), 3531. https://doi.org/10.3390/molecules24193531