Anti-Oxidant and Anti-Melanogenic Properties of Essential Oil from Peel of Pomelo cv. Guan Xi
Abstract
:1. Introduction
2. Results and Discussion
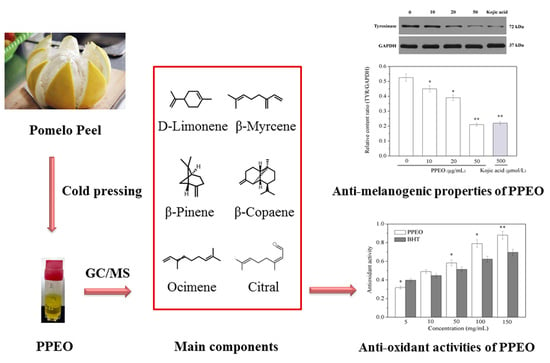
2.1. Chemical Composition of Pomelo Peel Essential Oil
2.2. Antioxidant Activities of Pomelo Peel Essential Oil
2.3. Anti-Melanogenic Effects of Pomelo Peel Essential Oil
2.3.1. Effect of Pomelo Peel Essential Oil on Cell Viability
2.3.2. Effect of Pomelo Peel Essential Oil on Cell Morphology
2.3.3. Inhibition of Pomelo Peel Essential Oil on Intracellular Tyrosinase Activity and Melanin Content
2.3.4. Effect of Pomelo Peel Essential Oil on Tyrosinase Expression in B16 Cells
2.4. Discussion
3. Materials and Methods
3.1. Materials
3.2. Extraction of Essential Oil
3.3. Gas Chromatography-Mass Spectrometry Analysis
3.4. Antioxidant Activities
3.4.1. DPPH Radical Scavenging Assay
3.4.2. Superoxide Anion Radical Scavenging Activity Assay
3.4.3. ABTS Radical Scavenging Assay
3.5. Cell Culture and Treatment
3.6. MTT Assay for Cell Viability
3.7. Immunofluorescence Analysis and Hoechst Staining
3.8. Determination of Melanin Content
3.9. Intracellular Tyrosinase Activity
3.10. Protein Extraction and Western Blot Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Liu, A.; Ibrahim, S.A.; Yang, H.; Huang, W. Isolation and characterization of microcrystalline cellulose from pomelo peel. Int. J. Biol. Macromol. 2018, 111, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, W.; Liao, X.; Hu, X.; Wu, J.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT Food Sci. Technol. 2017, 79, 640–646. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, Y.; You, L.; Shen, X.; Li, S. An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater. 2018, 241, 285–292. [Google Scholar] [CrossRef]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, N.B.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Wu, F.; Jin, Y.; Xu, X.; Yang, N. Electrofluidic pretreatment for enhancing essential oil extraction from citrus fruit peel waste. J. Clean. Prod. 2017, 159, 85–94. [Google Scholar] [CrossRef]
- Akhtar, M.; Iqbal, L.; Lateef, M.; Nawab, B.; Saleem, M.; Afza, N. Bio-reactive properties of citrus waste: An investigation of antioxidant and tyrosinase inhibitory activities. Pak. J. Bot. 2011, 43, 2881–2883. [Google Scholar]
- Fiocco, D.; Arciuli, M.; Arena, M.P.; Benvenuti, S.; Gallone, A. Chemical composition and the anti-melanogenic potential of different essential oils. Flavour Fragr. J. 2016, 31, 255–261. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-Scavenging Activities of Citrus Essential Oils and Their Components: Detection Using 1,1-Diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Dayanand, D.; Balamurugan, K.; Agrawal, R.C.; Rahul, V.; Rahi, J. Evalution of antioxidant activity of methanolic and hydromethanolic extract of sweet orange peels. Recent Res. Sci. Technol. 2011, 3, 22–25. [Google Scholar]
- Asikin, Y.; Maeda, G.; Tamaki, H.; Mizu, M.; Oku, H.; Wada, K. Cultivation line and fruit ripening discriminations of Shiikuwasha (Citrus depressa Hayata) peel oils using aroma compositional, electronic nose, and antioxidant analyses. Food Res. Int. 2015, 67, 102–110. [Google Scholar] [CrossRef]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Ritaro, M.; Hiroyuki, U.; Masayoshi, S. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Li, X.; Liu, X.H. Inhibitory effect of lemon essential oil on mushroom tyrosinase activity in vitro. Mod. Food Sci. Technol. 2015, 31, 97–105. [Google Scholar]
- Yang, C.H.; Huang, Y.C.; Tsai, M.L.; Chen, Y.C.; Li, L.L.; Ya, W.Y. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci. 2015, 37, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Phi, N.T.L.; Hung, P.V.; Chi, P.T.L.; Dung, N.H. Impact of Extraction Methods on Antioxidant and Antimicrobial Activities of Citrus Essential Oils. J. Essent. Oil Bear. Plants 2015, 18, 806–817. [Google Scholar] [CrossRef]
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Majid, N.M. Anti-elastase, anti-tyrosinase and matrix metalloproteinase-1 inhibitory activity of earthworm extracts as potential new anti-aging agent. Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl. 1), S348–S352. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Q.; Li, L.; Liu, J.; Shen, L.; Li, H.; Qin, W. Antioxidant activity and chemical compositions of essential oil and ethanol extract of Chuanminshen violaceum. Ind. Crops Prod. 2015, 76, 290–297. [Google Scholar] [CrossRef]
- Guo, J.J.; Gao, Z.P.; Xia, J.L.; Ritenour, M.A.; Li, G.Y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT Food Sci. Technol. 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Almulaiky, Y.; Zeyadi, M.; Saleh, R.; Baothman, O.; Al-shawafi, W.; Al-Talhi, H. Assessment of antioxidant and antibacterial properties in two types of Yemeni guava cultivars. Biocatal. Agric. Biotechnol. 2018, 16, 90–97. [Google Scholar] [CrossRef]
- Tu, P.T.; Tawata, S. Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef] [PubMed]
- Boskou, G.; Salta, F.N.; Chrysostomou, S.; Mylona, A.; Chiou, A.; Andrikopoulos, N.K. Antioxidant capacity and phenolic profile of table olives from the Greek market. Food Chem. 2006, 94, 558–564. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sall, C.; Dombrowsky, L.; Bottzeck, O.; Praud-Tabaries, A.; Blache, Y. Targeting bacterial biofilms: Design of a terpenoid-like library as non-toxic anti-biofilm compounds. Bioorg. Med. Chem. Lett. 2011, 21, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Donmez, E.; Unlu, M.; Candan, F.; Daferera, D.; Vardar-Unlu, G.; Polissiou, M.; Sokmen, A. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem. 2004, 84, 519–525. [Google Scholar] [CrossRef]
- Turina, A.V.; Nolan, M.V.; Zygadlo, J.A.; Perillo, M.A. Natural terpenes: Self-assembly and membrane partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Yao, Z.; Ma, Z.; Liu, J. Hypolipidemic effects of hickory nut oil using cold pressure extraction. Food Sci. Biotechnol. 2016, 25 (Suppl. 1), 41–46. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Pan, S.Y.; Fan, G.; Dong, L.; Ren, J.N.; Zhu, Y. Evaluation of volatile profile of Sichuan dongcai, a traditional salted vegetable, by SPME-GC-MS and E-nose. LWT Food Sci. Technol. 2015, 64, 528–535. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Wang, X.; Song, Y.; Fan, X.H.; Garcia Martin, J.F. Optimization of Pyrogallol Autoxidation Conditions and Its Application in Evaluation of Superoxide Anion Radical Scavenging Capacity for Four Antioxidants. J. AOAC Int. 2016, 99, 504–511. [Google Scholar] [CrossRef]
- Roberta, E.N.; Annap, R.; Ananth, P.; Annala, M.; Catherine, R.E. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Meira, W.V.; Heinrich, T.A.; Cadena, S.; Martinez, G.R. Melanogenesis inhibits respiration in B16-F10 melanoma cells whereas enhances mitochondrial cell content. Exp. Cell Res. 2017, 350, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Cerda, P.; Kim, H.J.; Wood, W.F.; Kubo, I. Effects of matsutake mushroom scent compounds on tyrosinase and murine B16-F10 melanoma cells. Biochem. Biophys. Res. Commun. 2017, 487, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Monika, P.; Claus, R.; Gudrun, M.; Donat, K.; Jochen, H.M. Ceramide-induced apoptosis of D283 medulloblastoma cells requires mitochondrial respiratory chain activity but occurs independently of caspases and is not sensitive to Bcl-Xl overexpression. J. Neurochem. 2002, 82, 424–494. [Google Scholar]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L.; Yang, C.H.; Chen, T.H.; Li, T.T.; Liu, L.L. Fenofibrate suppresses melanogenesis in B16-F10 melanoma cells via activation of the p38 mitogen-activated protein kinase pathway. Chem. Biol. Interact. 2013, 205, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Sun, Y.; Zhou, J.; Gu, Y.; Li, Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med. 2010, 25, 923–927. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |






| No. | Compound | Retention Index | Molecular Formula | Peak Area (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 951 | C10H16 | 0.15 |
| 2 | Artemisia triene | 966 | C10H6 | 0.05 |
| 3 | β-Pinene | 994 | C10H16 | 3.16 |
| 4 | β-Myrcene | 1009 | C10H16 | 31.17 |
| 5 | Limonene | 1050 | C10H16 | 55.92 |
| 6 | Ocimene | 1073 | C10H16 | 1.42 |
| 7 | Propionamide | 1149 | C9H11NO | 0.40 |
| 8 | Metaraminol | 1295 | C9H13NO2 | 0.21 |
| 9 | Citral | 1348 | C10H16O | 0.73 |
| 10 | 4-Carene | 1366 | C10H16 | 0.38 |
| 11 | Norephedrine | 1382 | C9H13NO | 0.04 |
| 12 | Caryophyllene | 1418 | C15H24 | 0.13 |
| 13 | Cubebene | 1463 | C15H24 | 0.15 |
| 14 | Cathinone | 1498 | C9H11NO | 0.05 |
| 15 | β-Copaene | 1516 | C15H24 | 1.24 |
| 16 | Bicyclogermacrene | 1523 | C15H24 | 0.24 |
| 17 | γ-Elemene | 1596 | C15H24 | 0.10 |
| 18 | 2,6,11,15-Tetramethyl-hexadeca-2,6,8,10,14-pentaene | 1979 | C20H30O2 | 0.50 |
| 19 | β-Farnesene | 2014 | C15H24 | 0.18 |
| 20 | 7-Methoxy-6-(3-methyl-2-oxobutyl)-2H-1-benzopyran-2-one | 2302 | C16H16O4 | 0.61 |
| 21 | 2-(Methylamino)-1-phenylethanol | 2329 | C9H13NO | 0.04 |
| Total | 96.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Li, X.; Peng, Y.; He, X.; Pan, S. Anti-Oxidant and Anti-Melanogenic Properties of Essential Oil from Peel of Pomelo cv. Guan Xi. Molecules 2019, 24, 242. https://doi.org/10.3390/molecules24020242
He W, Li X, Peng Y, He X, Pan S. Anti-Oxidant and Anti-Melanogenic Properties of Essential Oil from Peel of Pomelo cv. Guan Xi. Molecules. 2019; 24(2):242. https://doi.org/10.3390/molecules24020242
Chicago/Turabian StyleHe, Wanying, Xiaoyan Li, Ying Peng, Xiaoyan He, and Siyi Pan. 2019. "Anti-Oxidant and Anti-Melanogenic Properties of Essential Oil from Peel of Pomelo cv. Guan Xi" Molecules 24, no. 2: 242. https://doi.org/10.3390/molecules24020242
APA StyleHe, W., Li, X., Peng, Y., He, X., & Pan, S. (2019). Anti-Oxidant and Anti-Melanogenic Properties of Essential Oil from Peel of Pomelo cv. Guan Xi. Molecules, 24(2), 242. https://doi.org/10.3390/molecules24020242