CNF-Functionalization as Versatile Tool for Tuning Activity in Cellulose-Derived Product Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
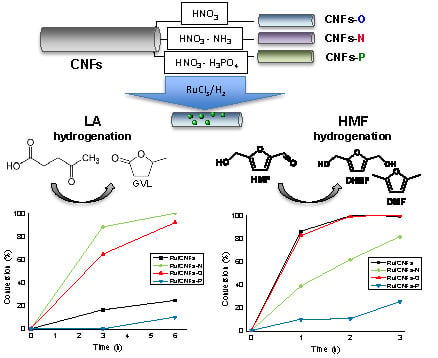
2.2. Catalytic Results—LA Hydrogenation
2.3. Catalytic Results—HMF Hydrogenation
3. Materials and Methods
3.1. Support Functionalization
3.2. Catalyst Preparation
3.3. LA Hydrogenation Reactions
3.4. HMF Hydrogenation Reactions
3.5. Catalysts Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edison, T.A. Manufacture of Carbon Filaments. U.S. Patent US484185A, 11 October 1892. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon nanofibers and their composites: A review of synthesizing, properties and applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef] [PubMed]
- De Jong, K.P.; Geus, J.W. Carbon Nanofibers: Catalytic Synthesis and Applications. Catal. Rev. Sci. Eng. 2000, 42, 481–510. [Google Scholar] [CrossRef] [Green Version]
- Coelho, N.M.A.; Furtado, J.L.B.; Pham-Huu, C.; Vieira, R. Carbon Nanofibers: A Versatile Catalytic Support. Mater. Res. 2008, 11, 353–357. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Chan-Thaw, C.E.; Campisi, S.; Veith, G.M.; Prati, L. The confinement effect on the activity of Au NPs in polyol oxidation. Catal. Sci. Technol. 2016, 6, 598–601. [Google Scholar] [CrossRef]
- Pan, X.; Fan, Z.; Chen, W.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 2007, 6, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Nhut, J.-M.; Nguyen, P.; Pham-Huu, C.; Keller, N.; Ledoux, M.-J. Carbon nanotubes as nanosized reactor for the selective oxidation of H2S into elemental sulfur. Catal. Today 2004, 91–92, 91–97. [Google Scholar] [CrossRef]
- Tessonnier, J.P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S.; et al. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon N. Y. 2009, 47, 1779–1798. [Google Scholar] [CrossRef] [Green Version]
- Prati, L.; Villa, A.; Chan-Thaw, C.E.; Arrigo, R.; Wang, D.; Su, D.S. Gold catalyzed liquid phase oxidation of alcohol: The issue of selectivity. Faraday Discuss. 2011, 152, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Wang, D.; Spontoni, P.; Arrigo, R.; Su, D.; Prati, L. Nitrogen functionalized carbon nanostructures supported Pd and Au–Pd NPs as catalyst for alcohols oxidation. Catal. Today 2010, 157, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Chan-Thaw, C.E.; Villa, A.; Veith, G.M.; Prati, L. Identifying the Role of N-Heteroatom Location in the Activity of Metal Catalysts for Alcohol Oxidation. ChemCatChem 2015, 7, 1338–1346. [Google Scholar] [CrossRef]
- Toebes, M.L.; Prinsloo, F.F.; Bitter, J.H.; Van Dillen, A.J.; De Jong, K.P. Influence of oxygen-containing surface groups on the activity and selectivity of carbon nanofiber-supported ruthenium catalysts in the hydrogenation of cinnamaldehyde. J. Catal. 2003, 214, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Toebes, M.L.; Zhang, Y.; Hájek, J.; Alexander Nijhuis, T.; Bitter, J.H.; Jos Van Dillen, A.; Murzin, D.Y.; Koningsberger, D.C.; De Jong, K.P. Support effects in the hydrogenation of cinnamaldehyde over carbon nanofiber-supported platinum catalysts: Characterization and catalysis. J. Catal. 2004, 226, 215–225. [Google Scholar] [CrossRef]
- Plomp, A.J.; Vuori, H.; Krause, A.O.I.; de Jong, K.P.; Bitter, J.H. Particle size effects for carbon nanofiber supported platinum and ruthenium catalysts for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2008, 351, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Campisi, S.; Sanchez Trujillo, F.; Motta, D.; Davies, T.; Dimitratos, N.; Villa, A. Controlling the Incorporation of Phosphorus Functionalities on Carbon Nanofibers: Effects on the Catalytic Performance of Fructose Dehydration. J. Carbon Res. 2018, 4, 9–26. [Google Scholar] [CrossRef]
- Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Gold Catalysts for the Selective Oxidation of Biomass-Derived Products. ChemCatChem 2019, 11, 309–318. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Su, D. 5-Hydroxymethylfurfural: A key intermediate for efficient biomass conversion. J. Energy Chem. 2015, 24, 548–551. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal Catalysts for the Efficient Transformation of Biomass-derived HMF and Furfural to Value Added Chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Hu, L.; Xu, J.; Zhou, S.; He, A.; Tang, X.; Lin, L.; Xu, J.; Zhao, Y. Catalytic Advances in the Production and Application of Biomass-Derived 2,5-Dihydroxymethylfuran. ACS Catal. 2018, 8, 2959–2980. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation processes: Advances on catalytic transformations of biomass-derived platform chemicals into hydrocarbon fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B. Levulinic acid hydrodeoxygenation, decarboxylation and oligmerization over NiMo/Al2O3 catalyst to bio-based value-added chemicals: Modelling of mass transfer, thermodynamics and micro-kinetics. Chem. Eng. J. 2017, 330, 383–397. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Trapp, M.A. The chemical conversion of biomass-derived saccharides: An overview. J. Braz. Chem. Soc. 2017, 28, 1586–1607. [Google Scholar] [CrossRef]
- Villa, A.; Chan-Thaw, C.E.; Campisi, S.; Bianchi, C.L.; Wang, D.; Kotula, P.G.; Kübel, C.; Prati, L. AuRu/AC as an effective catalyst for hydrogenation reactions. Phys. Chem. Chem. Phys. 2015, 17, 28171–28176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, A.; Schiavoni, M.; Chan-Thaw, C.E.; Fulvio, P.F.; Mayes, R.T.; Dai, S.; More, K.L.; Veith, G.M.; Prati, L. Acid-Functionalized Mesoporous Carbon: An Efficient Support for Ruthenium-Catalyzed γ-Valerolactone Production. ChemSusChem 2015, 8, 2520–2528. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of γ-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.R.H.; Palkovits, R. Development of Heterogeneous Catalysts for the Conversion of Levulinic Acid to γ-Valerolactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Daniel, R.; Ghafourian, A.; Herreros, J.M.; Shuai, S.; Ma, X. Combustion characteristics and emissions of 2-methylfuran compared to 2,5-dimethylfuran, gasoline and ethanol in a DISI engine. Fuel 2013, 103, 200–211. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J. A Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Jae, J.; Zheng, W.; Lobo, R.F.; Vlachos, D.G. Production of dimethylfuran from hydroxymethylfurfural through catalytic transfer hydrogenation with ruthenium supported on carbon. ChemSusChem 2013, 6, 1158–1162. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; De, S.; Saha, B. A brief summary of the synthesis of polyester building-block chemicals and biofuels from 5-hydroxymethylfurfural. Chempluschem 2012, 77, 259–272. [Google Scholar] [CrossRef]
- Bicker, M.; Kaiser, D.; Ott, L.; Vogel, H. Dehydration of d-fructose to hydroxymethylfurfural in sub- and supercritical fluids. J. Supercrit. Fluids 2005, 36, 118–126. [Google Scholar] [CrossRef]
- Gruter, G.J.M.; Dautzenberg, F. Method for the Synthesis of S-Alkoxymethyl Furfural Ethers and Their Use. U.S. Patent 8133289B2, 13 March 2012. [Google Scholar]
- Gruter, G.J.M.; Manzer, L.E.; De Sousa Dias, A.S.V.; Dautzenberg, F.; Purmova, J. Hydroxymethylfurfural Ethers and Esters Prepared in Ionic Liquids. U.S. Patent 8314260B2, 20 November 2012. [Google Scholar]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem. 2006, 8, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Nagpure, A.S.; Venugopal, A.K.; Lucas, N.; Manikandan, M.; Thirumalaiswamy, R.; Chilukuri, S. Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conversion of HMF to 2,5-dimethylfuran. Catal. Sci. Technol. 2015, 5, 1463–1472. [Google Scholar] [CrossRef]
- Zu, Y.; Yang, P.; Wang, J.; Liu, X.; Ren, J.; Lu, G.; Wang, Y. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ru/Co3O4 catalyst. Appl. Catal. B Environ. 2014, 146, 244–248. [Google Scholar] [CrossRef]
- Jae, J.; Mahmoud, E.; Lobo, R.F.; Vlachos, D.G. Cascade of liquid-phase catalytic transfer hydrogenation and etherification of 5-hydroxymethylfurfural to potential biodiesel components over Lewis acid zeolites. ChemCatChem 2014, 6, 508–513. [Google Scholar] [CrossRef]
- Hu, L.; Tang, X.; Xu, J.; Wu, Z.; Lin, L.; Liu, S. Selective transformation of 5-hydroxymethylfurfural into the liquid fuel 2,5-dimethylfuran over carbon-supported ruthenium. Ind. Eng. Chem. Res. 2014, 53, 3056–3064. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Piskun, A.S.; de Haan, J.E.; Wilbers, E.; van de Bovenkamp, H.H.; Tang, Z.; Heeres, H.J. Hydrogenation of Levulinic Acid to γ-Valerolactone in Water Using Millimeter Sized Supported Ru Catalysts in a Packed Bed Reactor. ACS Sustain. Chem. Eng. 2016, 4, 2939–2950. [Google Scholar] [CrossRef]
- Jalid, F.; Khan, T.S.; Mir, F.Q.; Haider, M.A. Understanding trends in hydrodeoxygenation reactivity of metal and bimetallic alloy catalysts from ethanol reaction on stepped surface. J. Catal. 2017, 353, 265–273. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Huš, M.; Likozar, B. Hydrogenation and hydrodeoxygenation of aromatic lignin monomers over Cu/C, Ni/C, Pd/C, Pt/C, Rh/C and Ru/C catalysts: Mechanisms, reaction micro-kinetic modelling and quantitative structure-activity relationships. Chem. Eng. J. 2019, 359, 305–320. [Google Scholar] [CrossRef]
- Cattaneo, S.; Naslhajian, H.; Somodi, F.; Evangelisti, C.; Villa, A.; Prati, L. Ruthenium on carbonaceous materials for the selective hydrogenation of HMF. Molecules 2018, 23, 2007. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.P.; Lin, L.; Liu, S. Synthesis of γ-valerolactone by hydrogenation of biomass- derivedLevulinic acid over Ru/C catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Gong, Y.; Zhang, P.; Li, H.; Wang, Y. Synthesis of palladium nanoparticles supported on mesoporous n-doped carbon and their catalytic ability for biofuel upgrade. J. Am. Chem. Soc. 2012, 134, 16987–16990. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Luo, J.; Yu, J.; Gorte, R.J.; Mahmoud, E.; Vlachos, D.G.; Smith, M.A. The effect of oxide acidity on HMF etherification. Catal. Sci. Technol. 2014, 4, 3074–3081. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.L.; Zhou, H.J.; Fu, Y. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Nitrogen-Doped Carbon-Supported Iron Catalysts. ChemSusChem 2016, 9, 1339–1347. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Sample | Atomic Ratio % C:N:O:P |
|---|---|
| CNFs | 97.1:0:2.9:0 |
| CNFs-N | 92.0:3.8:3.8:0 |
| CNFs-O | 85.2:0:14.8:0 |
| CNFs-P | 87.3:0:12.4:0.3 |
| Sample | N 1s | O 1s | P 1s | C 1s | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyridine | Pyrrole/PyRidone | Quaternary N+ | Pyridin Oxide | C=O, P=O, P-O | C-O, C-O-C, P-O-C | H2O | C-O-P | sp2 | sp3, C-P | C-O, C=O | C=C | ||
| CNFs-N | BE eV | 398.3 | 400.2 | 401.2 | 404.6 | 531.5 | 533.6 | 536.2 | - | 284.7 | 286.2 | 288.1 | 291.7 |
| Atom % | 49.3 | 44.1 | 3.5 | 3.1 | 43.4 | 53.2 | 3.4 | 73.1 | 13.4 | 8.8 | 4.8 | ||
| CNFs-O | BE eV | - | - | - | - | 531.3 | 533.1 | 534.5 | - | 284.6 | 285.1 | 288.4 | 291.5 |
| Atom % | 53.2 | 42.4 | 4.4 | 76.8 | 13.2 | 6.8 | 3.2 | ||||||
| CNFs-P | BE eV | - | - | - | - | 531.6 | 533.3 | 535.6 | 133.7 | 284.5 | 285 | 288.5 | 291.1 |
| Atom % | 50.1 | 44.5 | 5.4 | 100 | 77.4 | 14.4 | 5.7 | 2.5 | |||||
| Support | Pore Volume (mL g−1) |
|---|---|
| CNFs | 11.0 |
| CNFs-N | 7.2 |
| CNFs-O | 5.1 |
| CNFs-P | 8.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouve, A.; Cattaneo, S.; Capelli, S.; Stucchi, M.; Evangelisti, C.; Villa, A.; Prati, L. CNF-Functionalization as Versatile Tool for Tuning Activity in Cellulose-Derived Product Hydrogenation. Molecules 2019, 24, 316. https://doi.org/10.3390/molecules24020316
Jouve A, Cattaneo S, Capelli S, Stucchi M, Evangelisti C, Villa A, Prati L. CNF-Functionalization as Versatile Tool for Tuning Activity in Cellulose-Derived Product Hydrogenation. Molecules. 2019; 24(2):316. https://doi.org/10.3390/molecules24020316
Chicago/Turabian StyleJouve, Andrea, Stefano Cattaneo, Sofia Capelli, Marta Stucchi, Claudio Evangelisti, Alberto Villa, and Laura Prati. 2019. "CNF-Functionalization as Versatile Tool for Tuning Activity in Cellulose-Derived Product Hydrogenation" Molecules 24, no. 2: 316. https://doi.org/10.3390/molecules24020316
APA StyleJouve, A., Cattaneo, S., Capelli, S., Stucchi, M., Evangelisti, C., Villa, A., & Prati, L. (2019). CNF-Functionalization as Versatile Tool for Tuning Activity in Cellulose-Derived Product Hydrogenation. Molecules, 24(2), 316. https://doi.org/10.3390/molecules24020316