Fluoropolymer-Containing Opals and Inverse Opals by Melt-Shear Organization
Abstract
:1. Introduction
2. Results and Discussion
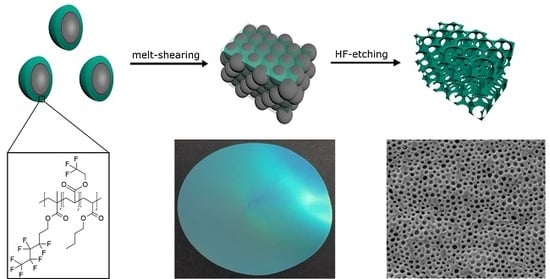
2.1. Bottom-Up Fabrication of Fluorine-Containing Opal and Inverse Opal Films
2.2. Optical Properties and Morphology of the PS@P(NFHMA-co-TFEA-co-nBuA) Opal Film
2.3. Chemical Resistance of the PS@P(NFHMA-co-TFEA-co-nBuA) Opal Film
2.4. Hydrophobicity and Solvent Response of the PS@P(NFHMA-co-TFEA-co-nBuA) Opal Film
2.5. Optical and Structural Properties of the SiO2@P(TFEA-co-NFHMA-co-iBuMA) Inverse Opal Film
2.6. Chemical Resistance and Solvent-Responsivness of the Inverse Opal Film
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of PS@PBuA@P(NFHMA-co-TFEA-co-nBuA) Core/Interlayer/Shell-Particles
3.3. Synthesis of SiO2@PBuA@P(TFEA-co-NFHMA-co-iBuMA) Core/Interlayer/Shell-Particles
3.4. Particle Processing and Preparation of Opal and Inverse Opal Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drobny, J.G. Technology of Fluoropolymers, 2nd ed.; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Munekata, S. Fluoropolymers as coating material. Progr. Org. Coat. 1988, 16, 113–134. [Google Scholar] [CrossRef]
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. Handbook of Fluoropolymer Science and Technology, 1st ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Progr. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Ameduri, B.; Boutevin, B. Well-Architectured Fluoropolymers: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Gardiner, J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem. 2015, 68, 13–22. [Google Scholar] [CrossRef]
- Moore, A.L. Fluoroelastomers Handbook: The Definitive User’s Guide and Databook; William Andrew Publishing: Norwich, NY, USA, 2005. [Google Scholar]
- Dams, R.; Hintzer, K. Chapter 1 Industrial Aspects of Fluorinated Oligomers and Polymers. In Fluorinated Polymers: Volume 2: Applications; The Royal Society of Chemistry: London, UK, 2017; Volume 2, pp. 1–31. [Google Scholar]
- Sun, W.; Zhou, S.; You, B.; Wu, L. A facile method for the fabrication of superhydrophobic films with multiresponsive and reversibly tunable wettability. J. Mater. Chem. A 2013, 1, 3146–3154. [Google Scholar] [CrossRef]
- Shi, F.; Song, Y.; Niu, J.; Xia, X.; Wang, Z.; Zhang, X. Facile Method to Fabricate a Large-Scale Superhydrophobic Surface by Galvanic Cell Reaction. Chem. Mater. 2006, 18, 1365–1368. [Google Scholar] [CrossRef]
- Feng, C.L.; Zhang, Y.J.; Jin, J.; Song, Y.L.; Xie, L.Y.; Qu, G.R.; Jiang, L.; Zhu, D.B. Reversible Wettability of Photoresponsive Fluorine-Containing Azobenzene Polymer in Langmuir-Blodgett Films. Langmuir 2001, 17, 4593–4597. [Google Scholar] [CrossRef]
- Pei, Y.; Travas-Sejdic, J.; Williams, D.E. Reversible electrochemical switching of polymer brushes grafted onto conducting polymer films. Langmuir 2012, 28, 8072–8083. [Google Scholar] [CrossRef]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. Res. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–550. [Google Scholar] [CrossRef]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Burns, A.; Kim, J.; Wiesner, U.; Hyeon, T. Designed Fabrication of Silica-Based Nanostructured Particle Systems for Nanomedicine Applications. Adv. Funct. Mater. 2008, 18, 3745–3758. [Google Scholar] [CrossRef]
- Phillips, K.R.; Vogel, N.; Hu, Y.; Kolle, M.; Perry, C.C.; Aizenberg, J. Tunable Anisotropy in Inverse Opals and Emerging Optical Properties. Chem. Mater. 2014, 26, 1622–1628. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Chen, J.; Lu, Z.; Yu, H.; Yan, Q.; Hng, H.H. Carbon inverse opal entrapped with electrode active nanoparticles as high-performance anode for lithium-ion batteries. Sci. Rep. 2013, 3, 2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, C.G.; Vowinkel, S.; Hellmann, G.P.; Herdt, T.; Contiu, C.; Schneider, J.J.; Gallei, M. A polymer based and template-directed approach towards functional multidimensional microstructured organic/inorganic hybrid materials. J. Mater. Chem. C 2014, 2, 7960–7975. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Gallei, M.; Zahn, J.T.; Engelhardt, J.; Hellmann, G.P.; Rehahn, M. Reversible Light-, Thermo-, and Mechano-Responsive Elastomeric Polymer Opal Films. Chem. Mater. 2013, 25, 2309–2318. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Biesalski, M.; Hellmann, G.P.; Rehahn, M.; Gallei, M. Paper-supported elastomeric opal films for enhanced and reversible solvatochromic response. J. Nanophotonics 2013, 7. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Winter, T.; Heidt, S.; Dietz, C.; Ding, T.; Baumberg, J.J.; Gallei, M. Smart polymer inverse-opal photonic crystal films by melt-shear organization for hybrid core–shell architectures. J. Mater. Chem. C 2015, 3, 2204–2214. [Google Scholar] [CrossRef] [Green Version]
- Schaffner, M.; England, G.; Kolle, M.; Aizenberg, J.; Vogel, N. Combining Bottom-Up Self-Assembly with Top-Down Microfabrication to Create Hierarchical Inverse Opals with High Structural Order. Small 2015, 11, 4334–4340. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.R.; England, G.T.; Sunny, S.; Shirman, E.; Shirman, T.; Vogel, N.; Aizenberg, J. A colloidoscope of colloid-based porous materials and their uses. Chem. Soc. Rev. 2016, 45, 281–322. [Google Scholar] [CrossRef] [Green Version]
- Gallei, M. Functional Polymer Opals and Porous Materials by Shear-Induced Assembly of Tailor-Made Particles. Macromol. Rapid Commun. 2018, 39, 1700648. [Google Scholar] [CrossRef] [PubMed]
- Schüth, F.; Schmidt, W. Microporous and Mesoporous Materials. Adv. Mater. 2002, 14, 629–638. [Google Scholar] [CrossRef]
- Stein, A. Advances in Microporous and Mesoporous Solids—Highlights of Recent Progress. Adv. Mater. 2003, 15, 763–775. [Google Scholar] [CrossRef]
- Thomas, A.; Goettmann, F.; Antonietti, M. Hard Templates for Soft Materials: Creating Nanostructured Organic Materials. Chem. Mater. 2008, 20, 738–755. [Google Scholar] [CrossRef]
- Llusar, M.; Sanchez, C. Inorganic and Hybrid Nanofibrous Materials Templated with Organogelators. Chem. Mater. 2008, 20, 782–820. [Google Scholar] [CrossRef]
- Joshi, R.K.; Schneider, J.J. Assembly of one dimensional inorganic nanostructures into functional 2D and 3D architectures. Synthesis, arrangement and functionality. Chem. Soc. Rev. 2012, 41, 5285–5312. [Google Scholar] [CrossRef] [PubMed]
- Scheid, D.; Cherkashinin, G.; Ionescu, E.; Gallei, M. Single-source magnetic nanorattles by using convenient emulsion polymerization protocols. Langmuir 2014, 30, 1204–1209. [Google Scholar] [CrossRef]
- Galisteo-López, J.F.; Ibisate, M.; Sapienza, R.; Froufe-Pérez, L.S.; Blanco, Á.; López, C. Self-assembled photonic structures. Adv. Mater. 2011, 23, 30–69. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.S.; Chang, W.S.; Kim, S.H. Liquid-impermeable inverse opals with invariant photonic bandgap. Adv. Mater. 2015, 27, 1282–1287. [Google Scholar] [CrossRef]
- Vogel, N.; Belisle, R.A.; Hatton, B.; Wong, T.S.; Aizenberg, J. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat. Commun. 2013, 4, 2167. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.; Wu, L. Fabrication of Robust Hydrophobic and Super-Hydrophobic Polymer Films with Onefold or Dual Inverse Opal Structures. Macromol. Mater. Eng. 2016, 301, 1430–1436. [Google Scholar] [CrossRef] [Green Version]
- Ruhl, T.; Spahn, P.; Hellmann, G.P. Artificial opals prepared by melt compression. Polymer 2003, 44, 7625–7634. [Google Scholar] [CrossRef]
- Finlayson, C.E.; Baumberg, J.J. Generating Bulk-Scale Ordered Optical Materials Using Shear-Assembly in Viscoelastic Media. Materials 2017, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Scheid, D.; Stock, D.; Winter, T.; Gutmann, T.; Dietz, C.; Gallei, M. The pivotal step of nanoparticle functionalization for the preparation of functional and magnetic hybrid opal films. J. Mater. Chem. C 2016, 4, 2187–2196. [Google Scholar] [CrossRef] [Green Version]
- Winter, T.; Su, X.; Hatton, T.A.; Gallei, M. Ferrocene-Containing Inverse Opals by Melt-Shear Organization of Core/Shell Particles. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef]
- Vowinkel, S.; Schäfer, C.G.; Cherkashinin, G.; Fasel, C.; Roth, F.; Liu, N.; Dietz, C.; Ionescu, E.; Gallei, M. 3D-ordered carbon materials by melt-shear organization for tailor-made hybrid core–shell polymer particle architectures. J. Mater. Chem. C 2016, 4, 3976–3986. [Google Scholar] [CrossRef] [Green Version]
- Vowinkel, S.; Malz, F.; Rode, K.; Gallei, M. Single-source macroporous hybrid materials by melt-shear organization of core-shell particles. J. Mater. Sci. 2017, 52, 11179–11190. [Google Scholar] [CrossRef]
- Vowinkel, S.; Boehm, A.; Schäfer, T.; Gutmann, T.; Ionescu, E.; Gallei, M. Preceramic Core-Shell Particles for the Preparation of Hybrid Colloidal Crystal Films by Melt-Shear Organization and Conversion into Porous Ceramics. Mater. Des. 2018, 160, 926–935. [Google Scholar] [CrossRef]
- Zhao, Q.; Finlayson, C.E.; Snoswell, D.R.E.; Haines, A.; Schäfer, C.; Spahn, P.; Hellmann, G.P.; Petukhov, A.V.; Herrmann, L.; Burdet, P.; et al. Large-scale ordering of nanoparticles using viscoelastic shear processing. Nat. Commun. 2016, 7, 11661. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, C.G.; Smolin, D.A.; Hellmann, G.P.; Gallei, M. Fully Reversible Shape Transition of Soft Spheres in Elastomeric Polymer Opal Films. Langmuir 2013, 29, 11275–11283. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.G.; Viel, B.; Hellmann, G.P.; Rehahn, M.; Gallei, M. Thermo-cross-linked Elastomeric Opal Films. ACS Appl. Mater. Interfaces 2013, 5, 10623–10632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, G.; Wang, Q.; Zhang, Q.; Zhan, X.; Chen, F. Novel Fluorinated Polymers Containing Short Perfluorobutyl Side Chains and Their Super Wetting Performance on Diverse Substrates. ACS Appl. Mater. Interfaces 2016, 8, 10513–10523. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; van Blaaderen, A. Metallodielectric Colloidal Core-Shell Particles for Photonic Applications. Langmuir 2002, 18, 524–534. [Google Scholar] [CrossRef]
- Schneider, H.A. Polymer class specificity of the glass temperature. Polymer 2005, 46, 2230–2237. [Google Scholar] [CrossRef]
- Viel, B.; Ruhl, T.; Hellmann, G.P. Reversible Deformation of Opal Elastomers. Chem. Mater. 2007, 19, 5673–5679. [Google Scholar] [CrossRef]
- Pursiainen, O.L.J.; Baumberg, J.J.; Winkler, H.; Viel, B.; Spahn, P.; Ruhl, T. Nanoparticle-tuned structural color from polymer opals. Opt. Express 2007, 15, 9553–9561. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Sild, S.; Karelson, M. Correlation and Prediction of the Refractive Indices of Polymers by QSPR. J. Chem. Inf. Comp. Sci. 1998, 38, 1171–1176. [Google Scholar] [CrossRef]
- Yao, W.; Li, Y.; Huang, X. Fluorinated poly(meth)acrylate: Synthesis and properties. Polymer 2014, 55, 6197–6211. [Google Scholar] [CrossRef] [Green Version]
- Gleinser, W.; Maier, D.; Schneider, M.; Weese, J.; Friedrich, C.; Honerkamp, J. Estimation of sphere-size distributions in two-phase polymeric materials from transmission electron microscopy data. J. Appl. Polym. Sci. 1994, 53, 39–50. [Google Scholar] [CrossRef]
- De Francisco, R.; Tiemblo, P.; Hoyos, M.; González-Arellano, C.; García, N.; Berglund, L.; Synytska, A. Multipurpose Ultra and Superhydrophobic Surfaces Based on Oligodimethylsiloxane-Modified Nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 18998–19010. [Google Scholar] [CrossRef] [Green Version]
- García-Domenech, R.; de Julián-Ortiz, J.V. Prediction of Indices of Refraction and Glass Transition Temperatures of Linear Polymers by Using Graph Theoretical Indices. J. Phys. Chem. B 2002, 106, 1501–1507. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Lederle, C.; Zentel, K.; Stuhn, B.; Gallei, M. Utilizing stretch-tunable thermochromic elastomeric opal films as novel reversible switchable photonic materials. Macromol. Rapid Commun. 2014, 35, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.J.; Terray, A.V. Refractive-index-driven separation of colloidal polymer particles using optical chromatography. Appl. Phys. Lett. 2003, 83, 5316–5318. [Google Scholar] [CrossRef]
- Aralaguppi, M.I.; Jadar, C.V.; Aminabhavi, T.M. Density, Viscosity, Refractive Index, and Speed of Sound in Binary Mixtures of Acrylonitrile with Methanol, Ethanol, Propan-1-ol, Butan-1-ol, Pentan-1-ol, Hexan-1-ol, Heptan-1-ol, and Butan-2-ol. J. Chem. Eng. Data 1999, 44, 216–221. [Google Scholar] [CrossRef]
- Awwad, A.M.; Al-Dujaili, A.H. Density, Refractive Index, Permittivity, and Related Properties for N-Formylmorpholine + Ethyl Acetate and + Butanone at 298.15 K. J. Chem. Eng. Data 2001, 46, 1349–1350. [Google Scholar] [CrossRef]
- Butt, H.J.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (particles, opals and inverse opals) are available from the authors. |










| Particle | DLS (d/nm) | TEM (d/nm) |
|---|---|---|
| PS | 206 ± 2 | 192 ± 10 |
| PS@P(nBuA-co-ALMA) | 211 ± 2 | 202 ± 6 |
| PS@P(NFHMA-co-TFEA-co-nBuA) | 246 ± 1 | 238 ± 11 |
| SiO2 | 240 ± 1 | 232 ± 6 |
| SiO2@MEMO | 249 ± 4 | 234 ± 11 |
| SiO2@P(nBuA-co-ALMA) | 271 ± 1 | 261 ± 8 |
| SiO2@P(TFEA-co-NFHMA-co-iBuMa) | 315 ± 9 | 303 ± 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kredel, J.; Dietz, C.; Gallei, M. Fluoropolymer-Containing Opals and Inverse Opals by Melt-Shear Organization. Molecules 2019, 24, 333. https://doi.org/10.3390/molecules24020333
Kredel J, Dietz C, Gallei M. Fluoropolymer-Containing Opals and Inverse Opals by Melt-Shear Organization. Molecules. 2019; 24(2):333. https://doi.org/10.3390/molecules24020333
Chicago/Turabian StyleKredel, Julia, Christian Dietz, and Markus Gallei. 2019. "Fluoropolymer-Containing Opals and Inverse Opals by Melt-Shear Organization" Molecules 24, no. 2: 333. https://doi.org/10.3390/molecules24020333
APA StyleKredel, J., Dietz, C., & Gallei, M. (2019). Fluoropolymer-Containing Opals and Inverse Opals by Melt-Shear Organization. Molecules, 24(2), 333. https://doi.org/10.3390/molecules24020333