Stability of Principal Hydrolysable Tannins from Trapa taiwanensis Hulls
Abstract
:1. Introduction
2. Results
2.1. Isolation of Hydrolysable Tannins from T. taiwanensis Hulls
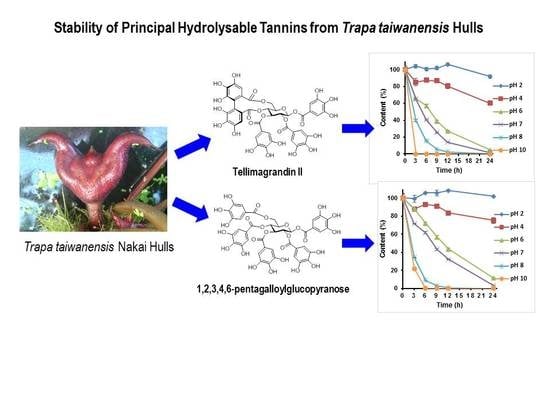
2.2. The Stability of Hydrlysable Tannins in Different pH Solutions
2.3. The Stability of Hydrlysable Tannins in Simulated Gastric Fluid and Intestinal Fluid
2.4. The Photostability of Hydrlysable Tannins
2.5. The Thermal Stability of Hydrlysable Tannins
2.6. Protective Effect of Ascorbic Acid on Thermal Stability
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Isolation of Hydrolysable Tannins from T. taiwanensis Hulls
4.4. Stability Studies
4.4.1. Preparation of Solutions
4.4.2. Stability of pH
4.4.3. Simulated Gastric Fluid Stability
4.4.4. Simulated Intestinal Fluid Stability
4.4.5. Photostability
4.4.6. Temperature Stability
4.4.7. Protective Effect of Ascorbic Acid Against Thermal Degradation
4.5. HPLC analysis of TGII and PGG
4.6. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Dall’Asta, M.; Calani, L.; Brighenti, F.; Del Rio, D. Gastrointestinal stability of urolithins: An in vitro approach. Eur. J. Nutr. 2017, 56, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, A.; Sundman, T. Stability and oxidation products of hydrolysable tannins in basic conditions detected by HPLC/DAD-ESI/QTOF/MS. Phytochem. Anal. 2013, 24, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Kao, M.Y.; Wu, S.C.; Lo, D.Y.; Wu, J.Y.; Chang, J.C.; Chiou, R.Y. Oral administration of Trapa taiwanensis Nakai fruit skin extracts conferring hepatoprotection from CCl4-caused injury. J. Agric. Food Chem. 2011, 59, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Subaiea, G.M.; Hussain, T.; Firdous, H. Hepatoprotective evaluation of Trapa natans against drug-induced hepatotoxicity of antitubercular agents in rats. Pharm. Mag. 2018, 14, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yang, C.; Wang, Y.; Yang, Y.; Yu, J. Antioxidant and antiproliferative activities of the leaf extracts from Trapa bispinosa and active components. S. Afr. J. Bot. 2017, 113, 377–381. [Google Scholar] [CrossRef]
- Lee, D.; Lee, O.H.; Choi, G.; Kim, J.D. Antioxidant and anti-adipogenic activities of Trapa japonica shell extract cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 327–334. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Seo, I.-B.; Jang, J.-H.; Son, S.; Jeong, J.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Characterization of the antioxidant fraction of Trapa japonica pericarp and its hepatic protective effects in vitro and in vivo. Food Funct. 2016, 7, 1689–1699. [Google Scholar] [CrossRef]
- Yu, H.; Shen, S. Phenolic composition, antioxidant, antimicrobial and antiproliferative activities of water caltrop pericarps extract. LWT-Food Sci. Technol. 2015, 61, 238–243. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Kwon, H.J.; Hong, H.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant activity and protective effects of Trapa japonica pericarp extracts against tert-butylhydroperoxide-induced oxidative damage in Chang cells. Food Chem. Toxicol. 2014, 64, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Kuroki, E.; Toyoda, M. Antioxidative compounds contained in the seed with hard shell of Trapa japonica Flerov. and its herbal tea. Nippon Kasei Gakkaishi 2013, 64, 353–359. [Google Scholar]
- Malviya, N.; Jain, S.; Jain, A.; Jain, S.; Gurjar, R. Evaluation of in vitro antioxidant potential of aqueous extract of Trapa natans L. fruits. Acta Pol. Pharm. 2010, 67, 391–396. [Google Scholar] [PubMed]
- Chiang, P.-Y.; Ciou, J.-Y. Effect of pulverization on the antioxidant activity of water caltrop (Trapa taiwanensis Nakai) pericarps. LWT-Food Sci. Technol. 2009, 43, 361–365. [Google Scholar] [CrossRef]
- Chiang, P.-Y.; Ciou, J.-Y.; Hsieh, L.-C. Antioxidant activity of phenolic compounds extracted from fresh and dried water caltrop pulp (Trapa taiwanensis Nakai). J. Food Drug Anal. 2008, 16, 66–73. [Google Scholar]
- Ciou, J.Y.; Wang, C.R.; Chen, J.C.; Chiang, P.Y. Total Phenolics content and antioxidant activity of extracts from dried water caltrop (Trapa Taiwanensis Nakai) hulls. J. Food Drug Anal. 2008, 16, 41–47. [Google Scholar]
- Radojevic, I.D.; Vasic, S.M.; Dekic, M.S.; Radulovic, N.S.; Delic, G.T.; Durdevic, J.S.; Comic, L.R. Antimicrobial and Antibiofilm Effects of Extracts from Trapa Natans L. Evaluation of total phenolic and flavonoid contents and GC-MS Analysis. Acta Pol. Pharm. 2016, 73, 1565–1574. [Google Scholar]
- Rahman, M.M.; Mosaddik, M.A.; Wahed, M.I.I.; Haque, M.E. Antimicrobial activity and cytotoxicity of Trapa bispinosa. Fitoterapia 2000, 71, 704–706. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hwang, J.-W.; Jang, J.-H.; Son, S.; Seo, I.-B.; Jeong, J.-H.; Kim, E.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Trapa japonica pericarp extract reduces LPS-induced inflammation in macrophages and acute lung injury in mice. Molecules 2016, 21, 392. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.E.; Choi, B.-K.; Kim, H.-S. Anti-inflammatory effects of water chestnut extract on cytokine responses via nuclear factor-κB-signaling pathway. Biomol. Ther. 2015, 23, 90–97. [Google Scholar] [CrossRef]
- Huang, H.C.; Chao, C.L.; Liaw, C.C.; Hwang, S.Y.; Kuo, Y.H.; Chang, T.C.; Chao, C.H.; Chen, C.J.; Kuo, Y.H. Hypoglycemic constituents isolated from Trapa natans L. Pericarps. J. Agric. Food Chem. 2016, 64, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, C.; Sarwar Alam, M.; Hamid, H.; Bano, S.; Haider, S.; Nazreen, S.; Ali, Y.; Javed, K. Trapa natans L. root extract suppresses hyperglycemic and hepatotoxic effects in STZ-induced diabetic rat model. J. Ethnopharmacol. 2014, 151, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Rosati, L.; Castagna, R.; Carloni, P.; Greci, L. Changes in ultraviolet absorbance and hence in protective efficacy against lipid peroxidation of organic sunscreens after UVA irradiation. J. Photochem. Photobiol. B Biol. 2006, 82, 204–213. [Google Scholar] [CrossRef] [PubMed]
- U.S. Pharmacopeia National Formulary USP38 NF33; The United States Pharmacopeial Convention: Rockviie, MD, USA, 2015; Volume 1, pp. 1884–1889. ISBN 978-1-936424-32-0.
Sample Availability: Samples of the compounds are available from the authors. |






| Irradiation time (h) | Content (%) | |
|---|---|---|
| 0 | 4 | |
| TGII | 100.00 ± 0.08 | 66.02 ± 1.60 |
| TGII + BHA | 100.00 ± 0.11 | 63.27 ± 0.37 |
| TGII + BHT | 100.00 ± 0.19 | 64.64 ± 0.15 |
| TGII + propyl gallate | 100.00 ± 1.16 | 51.62 ± 0.54 ** |
| TGII + catechin | 100.00 ± 0.44 | 57.12 ± 0.30 ** |
| PGG | 100.00 ± 3.15 | 70.79 ± 2.92 |
| PGG + BHA | 100.00 ± 0.41 | 66.40 ± 0.91 |
| PGG + BHT | 100.00 ± 4.27 | 52.80 ± 1.03 |
| PGG + propyl gallate | 100.00 ± 0.08 | 78.91 ± 2.20 ** |
| PGG + catechin | 100.00 ± 0.28 | 77.43 ± 2.17 ** |
| Ascorbic Acid (mg/mL) | TGII Content (%) 1 | |||
| 4 °C | 25 °C | |||
| 0 | 1.0 | 0 | 1.0 | |
| Time (week) | ||||
| 0 | 100.00 ± 1.02 | 100.00 ± 0.26 | 100.00 ± 1.02 | 100.26 ± 0.26 |
| 1 | 101.15 ± 0.35 | 98.31 ± 0.19 | 85.24 ± 0.69 | 83.75 ± 0.73 |
| 2 | 100.05 ± 0.80 | 95.78 ± 0.50 | 77.37 ± 1.57 | 75.56 ± 0.66 |
| 3 | 97.07 ± 0.71 | 92.88 ± 0.13 | 71.86 ± 0.84 | 68.18 ± 0.91 |
| 4 | 96.41 ± 1.51 | 90.92 ± 0.47 | 74.29 ± 0.60 | 52.54 ± 0.49 |
| Ascorbic Acid (mg/mL) | PGG Content (%) 1 | |||
| 4 °C | 25 °C | |||
| 0 | 1.0 | 0 | 1.0 | |
| Time (week) | ||||
| 0 | 100.00 ± 0.66 | 100.00 ± 0.25 | 100.00 ± 0.66 | 100.00 ± 0.25 |
| 1 | 100.30 ± 0.50 | 97.83 ± 0.06 | 95.89 ± 1.42 | 73.76 ± 0.31 |
| 2 | 100.13 ± 0.57 | 92.93 ± 0.81 | 93.47 ± 0.85 | 73.23 ± 0.47 |
| 3 | 100.35 ± 0.74 | 91.89 ± 0.74 | 86.82 ± 0.69 | 44.57 ± 0.11 |
| 4 | 104.18 ± 1.37 | 97.50 ± 0.43 | 91.82 ± 15.1 | 58.05 ± 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Chen, H.-F.; Wu, J.-Y.; Chen, L.-G. Stability of Principal Hydrolysable Tannins from Trapa taiwanensis Hulls. Molecules 2019, 24, 365. https://doi.org/10.3390/molecules24020365
Wang C-C, Chen H-F, Wu J-Y, Chen L-G. Stability of Principal Hydrolysable Tannins from Trapa taiwanensis Hulls. Molecules. 2019; 24(2):365. https://doi.org/10.3390/molecules24020365
Chicago/Turabian StyleWang, Ching-Chiung, Hsyeh-Fang Chen, Jin-Yi Wu, and Lih-Geeng Chen. 2019. "Stability of Principal Hydrolysable Tannins from Trapa taiwanensis Hulls" Molecules 24, no. 2: 365. https://doi.org/10.3390/molecules24020365
APA StyleWang, C.-C., Chen, H.-F., Wu, J.-Y., & Chen, L.-G. (2019). Stability of Principal Hydrolysable Tannins from Trapa taiwanensis Hulls. Molecules, 24(2), 365. https://doi.org/10.3390/molecules24020365