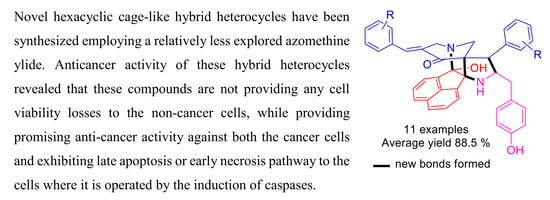
Design, Synthesis and In Vitro Mechanistic Investigation of Novel Hexacyclic Cage-Like Hybrid Heterocycles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. Chemistry
3.1.1. Hexacyclic Cage-Like Hybrid Heterocycle (4a)
3.1.2. Hexacyclic Cage-Like Hybrid Heterocycle (4b)
3.1.3. Hexacyclic Cage-Like Hybrid Heterocycle (4c)
3.1.4. Hexacyclic Cage-Like Hybrid Heterocycle (4d)
3.1.5. Hexacyclic Cage-Like Hybrid Heterocycle (4e)
3.1.6. Hexacyclic Cage-Like Hybrid Heterocycle (4f)
3.1.7. Hexacyclic Cage-Like Hybrid Heterocycle (4g)
3.1.8. Hexacyclic Cage-Like Hybrid Heterocycle (4h)
3.1.9. Hexacyclic Cage-Like Hybrid Heterocycle (4i)
3.1.10. Hexacyclic Cage-Like Hybrid Heterocycle (4j)
3.1.11. Hexacyclic Cage-Like Hybrid Heterocycle (4k)
3.2. Biology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 13 January 2019).
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics. CA 2012, 65, 87–108. [Google Scholar]
- Cooper, G.M. Cell Proliferation in Development and Differentiation. In The Cell: A Molecular Approach, 2nd ed.; ASM Press: Washington, DC, USA, 2000. [Google Scholar]
- Calvert, H. Cancer Drug Design and Discovery; Academic Press: San Diego, CA, USA, 2014; pp. xi–xiii. [Google Scholar]
- Reed, J.C. Dysregulation of apoptosis in cancer. J. Clin. Oncol. 1999, 17, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Tomaselli, K.J. Drug discovery opportunities from apoptosis research. Curr. Opin. Biotechnol. 2000, 11, 586–592. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.J. Targeting receptor tyrosine kinase MET in cancer: Small molecule inhibitors and clinical progress. J. Med. Chem. 2014, 57, 4427–4453. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Y.; Lin, X.; Ma, W.; Chen, G.; Li, W.; Wang, X.; Yu, Z. Platinum(iv) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance. Chem. Commun. 2018, 54, 5369–5372. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Wang, L.; Guo, T.; Wu, J.; Wan, J.; Zhou, L.; Li, H.; Li, Z.; Jiang, D.; et al. New Generation Nanomedicines Constructed from Self-Assembling Small-Molecule Prodrugs Alleviate Cancer Drug Toxicity. Cancer Res. 2017, 77, 6963–6974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Alshahrani, W.S.; Kotresha, D.; Altaf, M.; Azam, M.; Menéndez, J.C. Highly functionalized pyrrolidine analogues: Stereoselective synthesis and caspase-dependent apoptotic activity. RSC Adv. 2018, 8, 41226–41236. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kotresha, D.; Menéndez, J.C. Spirooxindole-pyrrolidine heterocyclic hybrids promotes apoptosis through activation of caspase-3. Bioorg. Med. Chem. 2019, 27, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.B.; Xu, H.X. Caged Garcinia xanthones: Development since 1937. Curr. Med. Chem. 2009, 16, 3775–3796. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zhan, X.K.; Yu, H.; Xie, G.R.; Wang, Z.Z.; Xiao, W.; Wang, Y.G.; Xiong, F.X.; Hu, J.F.; Yang, L.; et al. An open-labeled, randomized, multicenter phase IIa study of gambogic acid injection for advanced malignant tumors. Chin. Med. J. 2013, 126, 1642–1646. [Google Scholar] [PubMed]
- Wang, J.; Zhao, L.; Hu, Y.; Guo, Q.; Zhang, L.; Wang, X.; Li, N.; You, Q. Studies on chemical structure modification and biology of a natural product, gambogic acid (I): Synthesis and biological evaluation of oxidized analogues of gambogic acid. Eur. J. Med. Chem. 2009, 44, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Padmanilayam, M.P.; Puthucode, R.N.; Nazarali, A.J.; Motaganahalli, N.L.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Kraatz, H.B.; Prisciak, J.S.; et al. A conformational and structure-activity relationship study of cytotoxic 3,5-bis(arylidene)-4-piperidones and related Nacryloyl analogues. J. Med. Chem. 2001, 44, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Altaf, M.; Menéndez, J.C.; Kumar, R.R.; Osman, H. A Sustainable Approach to the Stereoselective Synthesis of Diazaheptacyclic Cage Systems Based on a Multicomponent Strategy in an Ionic Liquid. Molecules 2016, 21, 165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Menéndez, J.C.; Osman, H.; Kumar, R.R. Dipolar Cycloaddition-Based Multicomponent Reactions in Ionic Liquids: A Green, Fully Stereoselective Synthesis of Novel Polycyclic Cage Systems with the Generation of Two New Azaheterocyclic Rings. Synthesis 2015, 47, 2721–2730. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Osman, H.; Ali, M.A.; Basiri, A.; Kia, Y. An expedient synthesis and screening for antiacetylcholinesterase activity of piperidine embedded novel pentacyclic cage compounds. Med. Chem. 2014, 10, 228–236. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4(a–k) are available from the authors. |











| Entry | Compound | IC50 Values (µM, 24 h) | IC50 Values (µM, 48 h) | ||
|---|---|---|---|---|---|
| A549 | Jurkat | A549 | Jurkat | ||
| 1 |  | 21.89 ± 2.1 | 24.8 ± 3.1 | 14.9 ± 3.0 | 23.8 ± 2.8 |
| 2 |  | 65.45 ± 4.9 | 55.5 ± 4.4 | 44.7 ± 3.7 | 36.2 ± 4.2 |
| 3 |  | 93.08 ± 7.1 | 91.9 ± 6.8 | 58.9 ± 3.7 | 51.4 ± 4.6 |
| 4 |  | 83.78 ± 4.7 | 85.4 ± 4.8 | 74.6 ± 4.1 | 41 ± 3.4 |
| 5 |  | 71.6 ± 3.6 | 82.2 ± 2.5 | 70.8 ± 2.2 | 51 ± 6.8 |
| 6 |  | 72.6 ± 4.1 | 90.4 ± 2.4 | 65 ± 2.6 | 53.4 ± 8.9 |
| 7 |  | 66.2 ± 3.5 | 73.7 ± 4.4 | 59.1 ± 3.4 | 80.1 ± 3.5 |
| 8 |  | 75.5 ± 3.8 | 68.4 ± 4 | 62.5 ± 4.6 | 44.9 ± 3.2 |
| 9 |  | 63.6 ± 4.2 | 69.7 ± 3.7 | 52.8 ± 2.4 | 61.3 ± 3.5 |
| 10 |  | 77.3 ± 3.8 | 82.7 ± 2.9 | 66.4 ± 3.8 | 58 ± 3.8 |
| 11 |  | 50.5 ± 3.7 | 50.2 ± 8.2 | 31.9 ± 4.9 | 35.3 ± 2.1 |
| 12 | CPT | 40.55 ± 2.4 | 38.5 ± 3.1 | 34.1 ± 3.6 | 31.5 ± 4.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, S.K.; Almansour, A.I.; Natarajan, A.; Mohammad, F. Design, Synthesis and In Vitro Mechanistic Investigation of Novel Hexacyclic Cage-Like Hybrid Heterocycles. Molecules 2019, 24, 3820. https://doi.org/10.3390/molecules24213820
Raju SK, Almansour AI, Natarajan A, Mohammad F. Design, Synthesis and In Vitro Mechanistic Investigation of Novel Hexacyclic Cage-Like Hybrid Heterocycles. Molecules. 2019; 24(21):3820. https://doi.org/10.3390/molecules24213820
Chicago/Turabian StyleRaju, Suresh Kumar, Abdulrahman I. Almansour, Arumugam Natarajan, and Faruq Mohammad. 2019. "Design, Synthesis and In Vitro Mechanistic Investigation of Novel Hexacyclic Cage-Like Hybrid Heterocycles" Molecules 24, no. 21: 3820. https://doi.org/10.3390/molecules24213820
APA StyleRaju, S. K., Almansour, A. I., Natarajan, A., & Mohammad, F. (2019). Design, Synthesis and In Vitro Mechanistic Investigation of Novel Hexacyclic Cage-Like Hybrid Heterocycles. Molecules, 24(21), 3820. https://doi.org/10.3390/molecules24213820