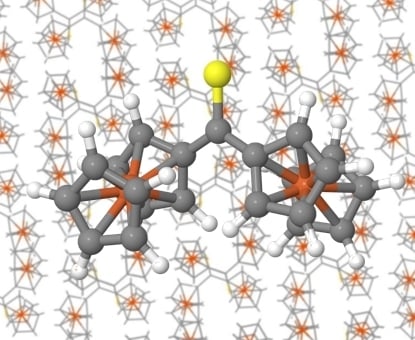
2.1. X-Ray Crystallography
In the experimental part of this work, the crystal structure of Fc
2CS was determined by its single-crystal XRD analysis. This analysis revealed that Fc
2CS crystallizes in the monoclinic space group
P2
1/
n. The asymmetric unit of the unit cell of Fc
2CS crystal contains one molecule. A summary of the crystal structure of Fc
2CS is given in
Table 1 and a single Fc
2CS molecule extracted from the crystal structure is shown in
Figure 1. The molecular structure of Fc
2CS exhibits two Fc-groups that are asymmetric with respect to one another. These groups are tilted in the opposite directions, relative to the C2–C1–C12 skeleton. To be precise, the Fc-groups are rotated by 19.4° and 20.1° out of the plane of the C2–C1–C12 skeleton. The cyclopentadienyl (Cp) rings within the Fc-groups are rotated merely by 1.6° and 5.3°, which indicates nearly eclipsed conformations of the Cp-rings.
It is instructive to compare the molecular structure of Fc
2CS with the structures determined for related crystalline compounds. To this end, a survey of the Cambridge Structural Database (CSD version 5.40, updates up to May 2019 [
33]) was conducted. Four crystal structures were found after a search for structures in which a Fc-group was bonded to a thiocarbonyl group and another substituent could be any moiety forming a C–C bond with this C=S group. These crystal structures are identified by the following CSD refcodes and chemical formulas: DUFYAG (C
18H
18Fe
1N
2O
1S
2) [
34], JEPVIJ (C
22H
14Cr
1Fe
1O
5S
1) [
35], VUTKAX (C
17H
12Fe
1O
5S
1W
1) [
36] and VUTKEB (C
29H
22Fe
2O
5S
1W
1) [
36]. Their molecular structures are shown in
Figure S1 in the
Supplementary Materials. The comparison of Fc
2CS with the Fc-functionalized thioketones present in the four reported structures indicates two important differences between their molecular structures. First, the Cp-rings of Fc
2CS are less rotated than those of the four related structures. The Cp-rings of JEPVIJ and VUTKEB exhibit a rotation angle of 20.7° and 18.7°, and therefore, they adopt intermediate conformations between staggered and eclipsed. The Cp-rings of DUFYAG and VUTKAX are rotated by 8.1° and 8.9°, respectively. Second, the Cp rings bonded to the C=S group of Fc
2CS show greater tilt angles outwards the C–C(=S)–C plane than the corresponding Cp-rings of DUFYAG and VUTKAX (their tilt angles are 5.0° and 9.1°, respectively). For JEPVIJ and VUTKEB, the Cp-ring bonded to their C=S group is practically co-planar with this group. A reason for these differences may be that Fc-functionalized thioketones readily act as ligands of transition metals, as it happens in the crystal structures JEPVIJ, VUTKAX and VUTKEB. The presence of other ligands and metal complexation via thiocarbonyl sulfur distort the geometry of these thioketones.
On the other hand, a marked similarity in molecular structure can be observed between Fc
2CS and its carbonyl analog (Fc
2CO). The crystal structure of the latter was deposited in the CSD with refcode FEOCKT01 [
37]. The Fc-groups of Fc
2CO are tilted in the opposite directions by 17.1° about the C–C(=O) bonds. The Cp-rings of Fc
2CO exhibit a nearly eclipsed conformation, with a rotation angle of 5.7°.
2.2. Choice of Computational Method
The theoretical part of this work started with establishing a proper computational methodology for studying Fc2CS. In order to achieve this, the performance of density functional theory (DFT), represented by the BP-D, BLYP-D, PBE-D, B97-D and TPSS-D functionals, was assessed in predicting the molecular and crystal structure of Fc2CS.
First, atomic positions in a single Fc
2CS molecule extracted from the XRD crystal structure were optimized using the five density functionals in combination with the sequence of three Karlsruhe “def2” basis sets of increasing size (from split valence to triple-ζ valence and to quadruple-ζ valence quality). The same starting geometry of the isolated Fc
2CS molecule was used in its optimization at all levels of theory.
Table 2 presents several geometrical parameters of the optimized molecule. For brevity, only results obtained from the PBE-D functional are shown in this table.
The optimized geometries were compared with the reference molecular geometry taken from the XRD crystal structure. To express the results of comparison quantitatively, the root-mean-square deviation (RMSD) of atomic positions in every optimized geometry relative to the reference geometry was calculated. The RMSD values for the Fc
2CS molecule optimized using all combinations of five density functionals and three basis sets are listed in
Table 3. The collation of RMSD values in each column reveals that the PBE-D functional consistently yields the lowest value. This means that the molecular structure predicted by PBE-D is the closest to the reference molecular geometry of Fc
2CS. The latter finding is in agreement with previous reports on the robustness of PBE-D in reproducing molecular geometries [
38,
39,
40]. The PBE-D geometry of Fc
2CS molecule shows very small deviations in angles and slightly less accurate bond lengths (
Tables S4–S6). The expansion of basis set from SVP to TZVP is associated with a marked improvement in the reproduction of reference geometry. Further increase of basis set size (that is, from TZVP to QZVP) has a negligible effect on the RMSD values. This proves that the molecular calculations converge fast toward the complete basis set limit. Similar convergence pattern in DFT calculations with TZVP and QZVP was previously detected for geometrical parameters of nitroanilines [
41]. As illustrated by the results in
Table 2, the experimental bond lengths are reproduced by PBE-D/TZVP with accuracy of 1–2 hundredths of Å. Such accuracy seems to be typical of DFT/TZVP calculations [
42].
To situate the aforementioned findings in a wider computational context, an isolated Fc
2CS molecule was also optimized using a correlated wave function theory (WFT) method. The SCS-MP2 method was selected because it improves the correlation energy of canonical MP2 theory [
43,
44,
45,
46,
47]. Although SCS-MP2 is one of the simplest and least computationally expensive correlated WFT methods, its computational times are much greater than those of DFT methods (formally
O(
N5) vs.
O(
N3)). The RMSD values yielded by SCS-MP2 are appended to
Table 3. They show that SCS-MP2 reproduces the reference molecular structure less accurately than the PBE-D method. Even though the SCS-MP2/SVP level luckily affords a RMSD value comparable to that of PBE-D/SVP, the SCS-MP2 method yields much greater RMSD values after the enlargement of basis set. Thus, this method predicts that the isolated Fc
2CS molecule shows a greater deviation from the molecular geometry observed in the crystal. The main reason for the great deviation of SCS-MP2 geometries is that the SCS-MP2 method systematically underestimates the distance between Fe and Cp-rings (
Table S7). This results from the inability of low-order perturbation theory to describe the bonding situation in FcH correctly [
48].
Next, the performance of the five density functionals in predicting the crystal structure of Fc
2CS was assessed. The XRD unit cell of Fc
2CS was used as a starting point for geometry optimization under periodic boundary conditions. Both the atomic positions and cell parameters of Fc
2CS were allowed to fully relax during the geometry optimization. The enormous computational cost of the optimization excluded the application of any basis sets larger than SVP. The calculated unit cells were compared with the XRD unit cell.
Table 4 presents two criteria that are taken into account for the assessment of the density functionals. The RMSD between the calculated and experimental atomic positions within the unit cell estimates the accuracy of the intrinsic geometry, orientation and alignment of molecules in the optimized crystal structure of Fc
2CS. The volume of unit cell is a criterion for judging the quality of optimized cell parameters. The RMSD values clearly indicate that the PBE-D functional describes the Fc
2CS molecules within the unit cell with the greatest accuracy. Again, this is in agreement with previous recommendations on the application of PBE-D to solid-state structures [
49,
50]. The main reason for the remarkable performance of PBE-D lies in its accurate description of intermolecular distances, leading to the proper orientation and alignment of Fc
2CS molecules. The RMSD values for individual Fc
2CS molecules occupying the unit cell prove that BLYP-D, PBE-D and TPSS-D are equally successful in reproducing the intrinsic geometry of Fc
2CS molecules. Thus, the orientation and alignment of Fc
2CS molecules within the unit cell are the deciding factors for the superior performance of PBE-D. The alignment of Fc
2CS molecules is associated with the quality of optimized cell parameters and, in consequence, the volume of unit cell. As shown in
Table 4, the PBE-D functional underestimates the volume of Fc
2CS unit cell merely by about 1%. A reduction by 2.0 to 8.0% occurs for the remaining density functionals. It should be stressed that some underestimation of unit cell volume is actually expected because our calculations neglected zero-point vibrations. It was previously reported that the calculated volume of unit cell for organic crystals is usually reduced by a range up to 2% due to the neglect of vibrational effects [
51]. The reduction in the PBE-D volume matches this range perfectly.
The results presented above confirm the credibility of PBE-D for predicting the structure of Fc
2CS and this functional is highly likely to succeed in reproducing other properties of Fc
2CS, such as its conformational behavior. There are indeed good grounds for such a supposition because PBE-D was successful in calculating conformational energies of other organic compounds [
52,
53]. Accordingly, PBE-D is used in the next part of this work. For comparison purposes, the SCS-MP2 method will be adopted in part of molecular calculations where it is computationally feasible.
2.3. Gas-Phase Structure
The key aspect of Fc
2CS molecular geometry is the mutual arrangement of two Fc-groups. They are bonded with the C=S group through single C–C bonds, which opens up an opportunity for their rotation. In consequence, rotational isomerism appears for the Fc
2CS molecule. To identify possible rotamers, the potential energy surface of an isolated Fc
2CS molecule was scanned along a coordinate representing the rotation of a single Fc-group about the adjacent C–C bond. The S1–C1–C2–C3 dihedral angle (
τFc) was selected to be this coordinate. The Cp-rings of each Fc-group displayed an eclipsed conformation in the starting geometries generated for the scan. The choice of this conformation will be justified further in this subsection. In each point of the scan, all geometrical parameters except
τFc were allowed to fully relax at the PBE-D/TZVP and SCS-MP2/TZVPP levels of theory. The scan is depicted in
Figure 2. To facilitate its analysis, the energies of optimized geometries are expressed relative to the lowest-energy geometry for which the relative energy (Δ
E) amounts to zero. It is easy to notice that there are four energy minima along the
τFc coordinate. Two of them (at around −170° and 150°) are equivalent due to symmetry reasons. All the minima fall into a very narrow range of ca. 1 kcal mol
−1. The structures corresponding to the minima possess their Cp-ring in nearly eclipsed conformations. Traversing the scan along
τFc is accompanied by the relaxation of another Fc-group; the S1–C1–C12–C16 angle varies between ca. −33° to ca. 29°. From
Figure 2 it can also be deduced that the Fc-rotation is associated with the occurrence of several energy barriers. Although the height of each barrier non-systematically varies with the level of theory, both levels agree that the highest barrier appears at
τFc of ca. −90°. Its height amounts to 10.6 and 12.3 kcal mol
−1 at the PBE-D/TZVP and SCS-MP2/TZVPP levels, respectively. Such a high barrier hinders the fast rotation of Fc-group. The occurrence of this barrier results from the adoption of perpendicular arrangement by the
π bonds of the C=S group and the adjacent Cp-ring of the revolving Fc-group. In this case another Cp-ring of the revolving Fc-group is oriented downward from the S1 atom. On the other hand, the barrier at around
τFc = 15° is small enough (ca. 2.5 kcal mol
−1) to allow for an easy interconversion between the neighboring rotamers.
Aside from the rotation of Fc-group as a whole, the internal rotation of Cp-rings within the Fc-group is also possible. With the aim of characterizing this kind of rotation, the potential energy surface of an isolated Fc
2CS molecule was scanned along a coordinate describing Cp-ring rotation within one of the Fc-groups. This coordinate was defined as the dihedral angle (
τCp) between C3, C11 and the centers of their Cp-rings. The
τCp angle was varied only from 0° to 72° due to the five-fold symmetry of Cp-ring. The resulting relaxed scan calculated at two levels of theory is presented in
Figure 3.
The energy minimum appears at
τCp of ca. 4° and the maximum is observed around 40°. The former corresponds to a nearly eclipsed conformation of Cp-rings and the latter is close to their staggered conformation. Our calculations indicated that the maximum is the transition state for Cp-ring rotation in Fc
2CS. This is in line with the experimental [
54,
55] and theoretical [
56,
57] findings on Cp-ring rotation in an isolated FcH molecule. The eclipsed conformation of Cp-rings in FcH is favored by 0.9 kcal mol
−1 [
55]. The energy barrier of Cp-ring rotation in Fc
2CS is estimated to be of 1.3 kcal mol
−1 at the PBE-D/TZVP level. Thus, this barrier is slightly higher than in FcH. The height of the barrier is severely overestimated by the SCS-MP2 method even though the spin scaling lowers this height, compared to that yielded by MP2 [
56].
The PBE-D/TZVP geometries corresponding to three non-equivalent minima in
Figure 2 were re-optimized at the PBE-D/QZVP level of theory. For the re-optimized rotamers, their harmonic vibrational frequencies were calculated at the same level of theory to confirm that the rotamers were true minima on the potential energy surface of Fc
2CS. The application of the QZVP basis set allowed us to practically eliminate the effect of basis-set incompleteness from the PBE-D results. The re-optimized rotamers are schematically depicted in
Figure 4 and they will be denoted by letters
A,
B and
C. The calculated geometrical, energetic and electric properties of
A–
C are summarized in
Table 5.
As shown in
Figure 4,
A and
C possess their Fc-groups tilted to the opposite directions, while
B displays its Fc-groups oriented in the same direction. All three rotamers lie close to each other in energy.
A is identified as the lowest-energy rotamer (Δ
E = 0). The tilt of both Fc-groups to the same direction destabilizes
B merely by 0.5 kcal mol
−1. The lowest stability is attributed to
C, which is 0.8 kcal mol
−1 higher in energy than
A. The addition of the corrections for the zero-point vibrational energy (ZPVE) to Δ
E do not change the sequence of rotamers’ stability
A >
B >
C. As evidenced by
τCp, the Cp-rings in the three rotamers adopt nearly eclipsed conformations. The stability of the rotamers is related to the magnitude of their dipole moment
μ. An increase in the stability is accompanied by the growing values of
μ.
The geometries exhibiting minimal energies in
Figure 2 were additionally re-optimized using SCS-MP2/QZVPP. This level of theory proves the occurrence of rotamers
A–
C and their selected parameters are appended to
Table 5. Both PBE-D/QZVP and SCS-MP2/QZVPP predict the same sequence of rotamers with respect to their stability. Moreover, both levels agree well in their Δ
E values of rotamers
B and
C.
Finally, it is necessary to compare the most stable rotamer in the gas phase with the crystalline geometry of Fc
2CS. Out of the three rotamers found in the gas phase,
A indeed bears the strongest resemblance to the crystalline geometry (see
Figure 4). The tilt of Fc-groups in the opposite directions changes only slightly between the gas phase and the crystal. Similarly, the Cp-rings of Fc-groups demonstrate nearly eclipsed conformations for both forms of Fc
2CS. To be precise, the distortions of
τFc and
τCp in
A from the corresponding values in the crystal do not exceed 2.2°.
Our calculations quite naturally predict that two Fc-groups of A are perfect mirror images but such molecular symmetry seems to be violated in the crystal. Nonetheless, the above comparison clearly proves that the transition of Fc2CS molecule from the gas phase to the crystal leads to a minor change in its geometry. This also indicates that the crystal packing affects the molecular geometry of Fc2CS only slightly.
2.4. Intramolecular Interactions
Even though the ΔE energies of gas-phase rotamers A–C fall into a very narrow range (<1 kcal mol−1), it is feasible to dissect main factors diversifying the stability of individual rotamers and, consequently, determining the molecular structure of Fc2CS. On a chemical basis, such factors may be understood as intramolecular interactions of different kinds. Here, the interactions occurring within the isolated molecule of Fc2CS were identified from distinct theoretical perspectives, such as orbital and topological ones, in order to provide an overall picture of factors affecting the molecular structure of Fc2CS.
An orbital-based viewpoint on intramolecular interactions was embodied in the analysis of molecular wavefunction in terms of localized orbitals of near-double occupancy. Accordingly, the natural bond order (NBO) analysis of
A–
C was performed and its results are summarized in
Table 6. First, the most accurate possible Lewis-like description of electron density was obtained for each rotamer, using the NBO method. Such a description assumed a localized assignment of electrons to bonding and non-bonding orbitals. The percentage of the total electron density not covered by the optimal Lewis-like description of each rotamer (%NL) is presented in
Table 6. %NL varies marginally from
A to
B, whereas the %NL value of
C is clearly smaller. This suggests that some corrections to the simple Lewis-like description have an important influence on the greater stability of
A and
B relative to
C. Such corrections include electron delocalizations from donor (occupied, Lewis-type) into acceptor (vacant, non-Lewis-type) NBOs. Second-order perturbative treatment of delocalization corrections allowed us to get a close look at the energetics of donor-acceptor delocalizations in
A–
C. The donor-acceptor
π→
π* delocalizations between each Fc-group and the C=S group turned out to be crucial for the relative stability of
A–
C.
Table 6 shows the energy (
E(2)) associated with these delocalizations. The negative sign of
E(2) indicates that the delocalizations are stabilizing. The
E(2)Fc→C=S* energy is attributed to all delocalizations from the donor π-type NBOs of a given Fc-group to the acceptor
π*-type NBO of the C=S group. The
E(2)C=S→Fc* energy is related to all delocalizations from the donor
π-type NBO of C=S to the acceptor
π*-type NBOs of Fc-group. The former always yields greater stabilization. Moreover, the magnitude of
E(2)Fc→C=S* indicates that the stabilizing effect of the Fc→C=S* delocalizations decreases in the sequence
A >
B >
C. This can be deduced from the
E(2)Fc→C=S* values in
Table 6 if these are summed up for
B and doubled for
A and
C. The sequence of rotamers ordered according to their total
E(2)Fc→C=S* values is identical to that established in the previous subsection. Thus, the
π→
π* delocalizations between the Fc-groups and the C=S group explain the relative stability of individual rotamers. These delocalizations can be essentially classified as “conjugative” in character, despite the non-coplanarity of the C=S group and the adjacent Cp-rings. The “conjugative” delocalizations are also manifested in the changes of bond lengths. The Fc→C=S* delocalizations lead to an elongation of C=S bond. That is why, the S1–C1 bond (1.666 Å,
Table 2) is longer than typical C=S bonds in alkyl thioketones, e.g., dimethyl thioketone shows the C=S bond length of 1.635 Å at the PBE-D/QZVP level. Furthermore, the C–C bonds linking the Fc-groups to the C=S group get compressed due to the “conjugative” delocalizations between these groups. That is why, the C1–C2 bond (1.464 Å,
Table 2) is shorter than typical C–C bonds in alkyl thioketones, e.g., dimethyl thioketone possesses the C–C bond lengths of 1.501 Å at the PBE-D/QZVP level.
Rotamers
A–
C are differentiated by the tilt of their Fc-groups and these bulky groups are at different distances from one another within
A–
C. The Fc-groups of
A and
B are in closer proximity than those of
C. Thus, the former should demonstrate greater steric repulsion. The NBO analysis also offers insights into the steric interactions between the Fc-groups of
A–
C.
Table 6 shows the steric exchange energy (
E(sx)) that quantifies pairwise steric interactions between filled orbitals. The repulsive character of these interactions is reflected by the positive values of
E(sx). The
E(sx)Fc)(Fc energy estimates the steric interactions between both Fc-groups of each rotamer. Its values indicate that the steric interactions have a smaller effect on the stability of
A–
C than the
π→
π* delocalizations have. As it was expected,
C is characterized by the smallest value of
E(sx)Fc)(Fc. The greatest repulsion between the Fc-groups occurs for
A. The leading pairwise steric exchange interaction between the Fc-groups involves the
σ-orbitals of their C–H bonds lying in close proximity, e.g., C6–H6 and C13–H13 for
A (
Figure 4). The
E(sx)C–H)(H–C energy shown in
Table 6 covers all steric interactions between such C–H bonds for each rotamer.
The interactions between the Fc-groups within
A–
C were subsequently examined from a perspective based on the topological analysis of electron density in real space. The quantum theory of atoms in molecules (QTAIM) was used to identify subtle interactions between the Fc-groups of each rotamer. QTAIM molecular graphs of
A–
C are plotted in
Figure 5. This figure proves that the close proximity of Fc-groups within
A and
B results in the occurrence of three bond paths linking the Fc-groups (black dashed lines in
Figure 5). Two bond paths are assigned to C···H interactions that resemble some unusual kind of hydrogen bonding but they cannot be classified as C–H···C(
π) [
58]. The third bond path corresponds to a H···H interaction. The molecular graph of
C reveals only a single bond path between two H atoms of Fc-groups. All these interactions were characterized in greater detail using various parameters calculated at the critical point on each aforementioned bond path (
Table S8). For these bond paths, their critical point always shows very low values of electron density, its Laplacian and total electron energy density. The Laplacian of electron density and the total electron energy density adopt positive values. Such values of the three parameters describe very weak, closed-shell interactions [
59]. Convincing evidence for the closed-shell nature of C···H and H···H interactions within
A–
C is additionally provided by other critical-point parameters (see footnotes to
Table S8) [
60,
61].
Our last step towards the detection of even more subtle intramolecular interactions was taken by using the non-covalent interaction (NCI) visualization index. The plots showing NCI isosurfaces for
A–
C are presented in
Figure 5. The isosurfaces are colored according to the sign of the second Hessian eigenvalue multiplied by the electron density (sign(
λ2)
ρ). Their blue regions illustrate the occurrence of attractive interactions, while their red regions identify repulsive interactions. The inspection of the NCI isosurfaces for
A–
C leads to several findings. First, the blue regions are found between atoms involved in the C···H and H···H interactions, which is in accordance with the QTAIM results. These blue regions border on red regions that in turn are responsible for steric congestion inside the pseudo-rings formed by the C···H and H···H interactions and the surrounding carbon skeleton [
31]. Second, the existence of weak stabilizing interactions between the lone electron pairs of S1 and the nearest H atoms of Cp-rings can be observed. The resulting S···H interactions are constrained by the tilt of Fc-groups. Third, there is a vast region, colored in green and yellow, between the Fc-groups of
B. This region signals extremely weak interactions between the
π-clouds of the Fc-groups.
2.5. Solvated Structure
Having established the structure of an isolated Fc
2CS molecule, we examine to what extent this structure suffers from the presence of a solvent. The examination of solvent effect is important due to the necessity of carrying out reactions with Fc
2CS in solution [
18]. Three solvents of different polarities, in terms of their dielectric constant and dipole moment, were considered here. In order of increasing polarity, tetrahydrofuran (THF), ethanol (EtOH) and acetonitrile (MeCN) were selected as the solvents. The conductor-like screening model (COSMO) was used to evaluate the effect of these solvents on the Fc
2CS molecule. In the case of Fc
2CS solvation by EtOH, we neglected possible hydrogen bonding interactions between the C=S group and one or more hydroxyl groups of EtOH.
The potential energy surface of a Fc
2CS molecule in the three solvents was scanned along the
τFc and
τCp coordinates at the PBE-D/TZVP level of theory. Except the dihedral angles corresponding to the two coordinates, all other geometrical parameters of solvated Fc
2CS molecule were allowed to fully relax. The resulting scans are plotted in
Figure 6 and
Figure 7. In these figures, the PBE-D/TZVP results for the isolated Fc
2CS molecule are repeated after
Figure 2 and
Figure 3. It is evident that the conformational behavior of the solvated molecule is fairly similar to that in the gas phase. The solvated molecule demonstrates the minima and maxima of its Δ
E energy at practically the same
τFc and
τCp angles as the isolated molecule does. By contrast, the solvent effect on the height of energy barriers is noticeable, particularly for the rotation of Fc-group. This effect grows with the increase of solvent polarity. However, the effect of a given solvent on the height of barriers for the Fc-group rotation is not uniform. For instance, the barrier at ca. 95° becomes higher by 14% whereas the barrier at ca. 15° is lowered by half upon solvation by MeCN (
Figure 6).
The height of the highest barrier remains practically unchanged if only the Fc-group rotation starts at the
τFc angle corresponding to rotamer
A. For the rotation of Cp-rings within the Fc-group, its barrier is increased slightly upon solvation (
Figure 7). The Cp-ring rotation for Fc
2CS solvated in MeCN is associated with clearing a barrier of 1.4 kcal mol
−1, which is merely 5.5% higher than in the gas phase.
Figure 7 indicates that the nearly eclipsed conformation of Cp-rings in Fc
2CS is still favored upon solvation. This is in agreement with the conformation of FcH in solutions. Experimental studies suggested the dominance of eclipsed conformer for FcH in solutions [
57,
62,
63].
For the solvated Fc
2CS molecule, its geometries representing the minima of Δ
E in
Figure 6 were subsequently re-optimized in three solvents at the PBE-D/QZVP level. The isolated Fc
2CS molecule could exist as a mixture of three rotamers
A–
C and the solvated molecule likewise. Selected parameters of
A–
C in three solvents are listed in
Table 7. The comparison of
τFc and
τCp in
Table 7 to those of the isolated Fc
2CS molecule (
Table 5) reveals that the solvation of Fc
2CS has a minimal effect on these angles. On closer inspection of
τFc and
τCp in
Table 7, it is clear that the angles change marginally with the increase of solvent polarity.
Bearing in mind that dihedral angles are softer than bond lengths and angles, it can be deduced that the geometrical parameters of a solvated Fc2CS molecule are almost insensitive to solvent polarity. The values of ΔE + ΔZPVE indicate that A is still designated as the preferred rotamer in all three solvents. Furthermore, no variations in the decreasing stability of rotamers A > B > C are observed after adding the solvents. It is worth noting that the preference of A is enhanced by the increasing polarity of solvents. There is a clear relation between the dipole moment of A–C and the solvent polarity. The values of μ become higher and higher for all rotamers but the μ value of A grows to the greatest extent. This is because A is the most polar rotamer out of A–C. In consequence, the strongest stabilization due to electrostatic solute-solvent forces occurs for solvated rotamer A.
2.6. Crystal Structure
The crystal structure of Fc
2CS was calculated at the PBE-D/SVP level of theory. Both the cell parameters and the atomic positions of all 168 atoms occupying the unit cell were optimized. The optimization of atomic positions included all atoms because the
P1 space-group symmetry was exploited in this optimization, due to the limitations of the current version of TURBOMOLE. Calculated geometrical parameters relevant for the crystal structure of Fc
2CS are summarized in
Table 8. The calculated lattice parameters are in close accordance with the values measured using the XRD method. The percentage variations of the calculated lattice parameters from the XRD values do not exceed ±0.5%. These variations result in a slightly too small volume of the computed unit cell (
Table 4). The contraction of unit cell is a well-known consequence of the neglect of ZPVE and thermal effects in periodic calculations [
64]. In comparison with the XRD structure, four Fc
2CS molecules occupying the optimized unit cell lie closer to each other. This movement of the Fc
2CS molecules closer to each other is illustrated by shorter minimal intermolecular distances (min
dinter) of various kind (e.g., H···H, C···H, S···H and C···C). A fundamental deviation of the calculated crystal structure from the XRD one is that the former shows the symmetry-independence of the four Fc
2CS molecules within the unit cell. This can obviously be explained by the exploitation of
P1 space-group symmetry in our periodic DFT calculations. Each Fc
2CS molecule exhibits unique
τFc and
τCp angles and, therefore, the ranges of
τFc and
τCp are given in
Table 8. Fortunately, these ranges are quite narrow. The comparison between the calculated and experimental values of
τFc and
τCp reveals that the PBE-D/SVP calculations yielded the crystal structure with somewhat larger asymmetry of Fc-groups in each Fc
2CS molecule.
Both the XRD analysis and the periodic DFT calculations prove that the Fc
2CS molecules in the crystal structure form a pattern with a highly characteristic arrangement of Fc-groups. Such an arrangement is based on the structure of a dimer comprised of two FcH molecules [
65]. The FcH molecules in such a dimer are oriented parallel to their axes running through the geometrical centers of their Cp-rings. Simultaneously, one of the molecules is shifted along the axis running through the geometrical centers of its Cp-rings. As a result of the shift, the Fe atom of each FcH molecule lies on the same plane with one of the Cp-rings of another FcH molecule. The FcH dimer was recognized as a common building block in the crystal structures of ferrocene derivatives [
65]. The analysis of the electrostatic potential calculated for the FcH dimer revealed a large stabilizing electrostatic complementarity between the FcH units of this dimer [
65]. In the case of Fc
2CS, the spatial organization of the Fc-groups belonging to neighboring molecules resembles that of the FcH dimer (
Figure 8). The calculated crystal structure indeed demonstrates a set of almost right angles formed by the geometrical centers of Cp-rings and their two nearest Fe atoms (one belonging to the same molecule and the other of neighboring molecule). Furthermore, two Fe atoms with their respective Cp-ring centers (linked with dashed lines in
Figure 8) lie almost on the same plane.
The calculated crystal structure can also be characterized in terms of energetic quantities. The central quantity to assess the stability of a crystal is its lattice energy. The lattice energy of Fc2CS calculated at the PBE-D/SVP level amounts to −40.9 kcal mol−1. Its negative value means that the crystal is stable with respect to dissociation into separated gas-phase molecules in their lowest-energy conformation. The effect of crystal packing on the molecular structure of Fc2CS can be expressed by the strain energy. For Fc2CS, the distortion of its molecular structure in the crystal from that in the gas phase increases the molecular energy by 1.1 kcal mol−1 at the PBE-D/SVP level of theory. This is the strain energy that must be compensated by intermolecular interactions in the crystal.
2.7. Intermolecular Interactions
For the optimized crystal structure of Fc
2CS, its electron density calculated at the PBE-D/SVP level of theory was examined using the QTAIM and NCI methods in order to detect intermolecular interactions occurring in the crystal of Fc
2CS. The QTAIM topological analysis of electron density located plenty of critical points in the intermolecular regions of the crystal structure. After the elimination of all ring and cage critical points, the remaining critical points described three kinds of intermolecular contacts: H···H, C···H and S···H.
Figure 9 shows a tiny fraction of the critical points corresponding to the three kinds of intermolecular contacts. Several QTAIM parameters were determined at the critical points of H···H, C···H and S···H contacts to characterize the interactions between the atoms forming these contacts. Irrespective of which contact is considered, the electron density at the critical point is very low in value (<0.01 a.u.) and the Laplacian of the electron density is also small and positive (<0.035 a.u.). The total electron energy density at the critical point is positive but it approaches zero (<0.0015 a.u.). Such values of the parameters testify that the intermolecular H···H, C···H and S···H contacts can be classified as closed-shell interactions and their strength is estimated to be very low. The shortest H···H contact is found at a distance of 2.156 Å (
Table 8) and the vast majority of such contacts are no longer than 2.8 Å. Thus, the Fc
2CS crystal presents slightly longer intermolecular H···H contacts than their typical range 2.18–2.57 Å [
66]. This indicates that for Fc
2CS there are no sufficiently strong crystal packing effects to give rise to short intermolecular H···H contacts and, in consequence, to severe steric overcrowding in the structure of Fc
2CS. The interatomic distances of S···H contacts in Fc
2CS lie in the range from 2.821 Å to 3.407 Å. The QTAIM analysis reveals that each S atom in the crystal structure of Fc
2CS can participate in more than one intermolecular S···H hydrogen bonding interaction. Most of these interactions are detected at longer distances (>3 Å) than typical intermolecular (C=)S···H hydrogen bonding contacts reported for molecular crystals [
67]. Formation of several (C=)S···H contacts with the same S atom is known for many crystal structures [
67]. For instance, a hydrogen bonding pattern composed of four S···H interactions to a thiocarbonyl group was observed in the crystal structure of thiourea [
68]. Based on previous theoretical studies of model complexes [
69,
70], a single S···H interaction is expected to be rather weak. However, the stabilizing effect of S···H interactions in Fc
2CS should be strengthened to a certain extent, remembering that a given S atom can be involved in more than one S···H interaction simultaneously. Out of the critical points describing the intermolecular contacts in the crystal structure of Fc
2CS, those corresponding to C···H interactions are scarcest. The C···H contact of 3.530 Å is in general the longest for which the critical point was detected in Fc
2CS.
The QTAIM-based picture of a network of weak H···H, C···H and S···H interactions between the molecules of Fc
2CS crystal is supported by the results of NCI analysis. The NCI isosurface generated for the optimized unit cell of Fc
2CS is plotted in
Figure 9. It is noticeable that the NCI isosurface extends over vast intermolecular regions where the H···H, C···H and S···H contacts appear. This part of the NCI isosurface is colored in green and it signals the occurrence of weak interactions. The lack of any blue domains for the NCI isosurface in the intermolecular regions indicates that no strong interactions are localized between the molecules of Fc
2CS. The blue and red domains of the NCI isosurface are observed within the Fc-groups. These domains originate from the close proximity of two aromatic rings, which leads to attractive and repulsive interactions between the
π-clouds of both Cp-rings.
The energetic analysis of the interactions between the molecules of Fc
2CS crystal was performed to provide a detailed insight into the strength and nature of physical forces lying behind these interactions. The intermolecular interaction energy (
Einter) was determined using a cluster approach to the crystal structure of Fc
2CS (
Figures S3–S5). Within this approach, the structural motif shown in
Figure 8 was represented by two Fc
2CS molecules forming a dimer. Its calculated
Einter energy amounts to −8.0 kcal mol
−1 at the PBE-D/SVP level of theory. The negative value of
Einter means a greater stability of the dimer relative to its molecular constituents. The magnitude of
Einter suggests that the dimer is rather weakly bound. Nonetheless, this magnitude is sufficient to compensate for the strain energy of both Fc
2CS molecules (2.2 kcal mol
−1 in total). The attraction between the molecules in fact far outweighs the increase of their intramolecular energy. The calculations of
Einter for various dimers extracted from the optimized crystal structure of Fc
2CS (
Table S9) lead to the conclusion that the structural motif from
Figure 8 is associated with particularly efficient stabilization.
The localized molecular orbital energy decomposition analysis (LMOEDA) was used to establish the importance of individual physical forces in the interactions between the molecules of Fc
2CS. LMOEDA results for a dimer representing the structural motif from
Figure 8 are summarized in
Table 9. The
Einter energy calculated at the PBE-D/SVP level is decomposed into the electrostatic (
Eelst), polarization (
Epol), dispersion (
Edisp) and exchange-repulsion (
Eexch-rep) components. It is clear that
Edisp is the dominant attractive component, while
Epol provides only 14.1% of the total attraction between the molecules. The LMOEDA method was subsequently used to decompose the
Einter energies of other dimers extracted from the optimized crystal structure of Fc
2CS (
Table S9). The
Edisp component turns out to be the dominant attractive component for all dimers. This component represents at least 56.1% of the total attraction between two Fc
2CS molecules. The dominant role of
Edisp is indeed expected for systems with highly polarizable electron densities, such as the
π-clouds of Fc-groups in our case. The
Epol component is always the smallest, providing at most 15.6% of the total attraction. In general, the more strongly two Fc
2CS molecules interact, the smaller percentage share of
Edisp is observed, and simultaneously, it is accompanied by the greater percentage share of
Eelst. Therefore, two Fc
2CS molecules forming the structural motif from
Figure 8 demonstrate the highest percentage share of
Eelst among all dimers studied. This is also in line with the finding on the large stabilizing electrostatic complementarity in the FcH dimer [
65], as it was mentioned in the previous subsection.
Finally, the many-body analysis of
Einter calculated for larger clusters of Fc
2CS molecules was performed to dissect the energetic effects beyond the interactions within the pairs of Fc
2CS molecules. A series of clusters composed of three Fc
2CS molecules was extracted from the optimized crystal structure of Fc
2CS. The
Einter energy of each trimer was calculated at the PBE-D/SVP level of theory and then partitioned into two- and three-body contributions (
Table S10). Here, the “body” refers to a single Fc
2CS molecule. Thus, the two-body contribution covers the interactions for all pairs of Fc
2CS molecules within the trimer. The three-body contribution quantifies the effect of a third molecule on the interaction between the other two molecules within the trimer. Unsurprisingly, the two-body contribution is mainly responsible for the stability of all trimers considered. The three-body contribution accounts for 4.3–17.6% of
Einter. The many-body analysis of
Einter for a cluster of four Fc
2CS molecules (that is, a tetramer) produced a negligible four-body contribution. This indicates that the higher-body contributions diminished quickly with the growing number of Fc
2CS molecules.